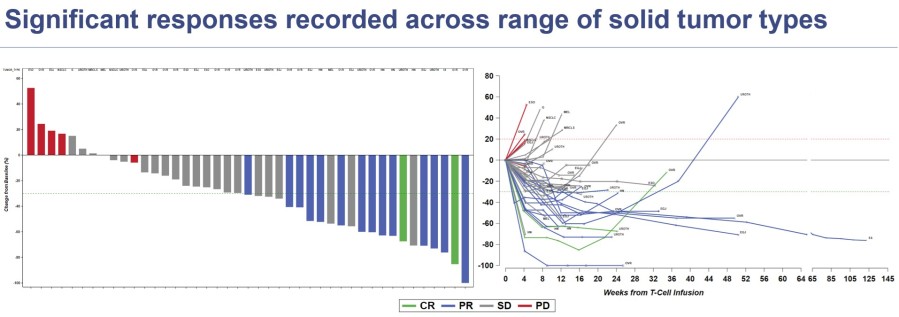
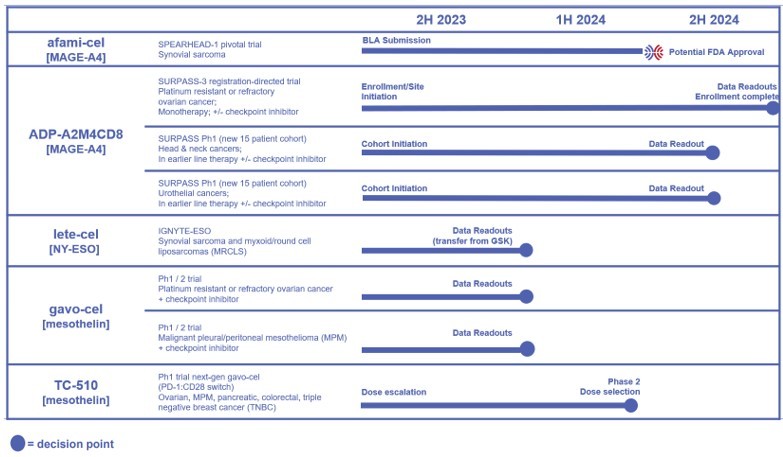
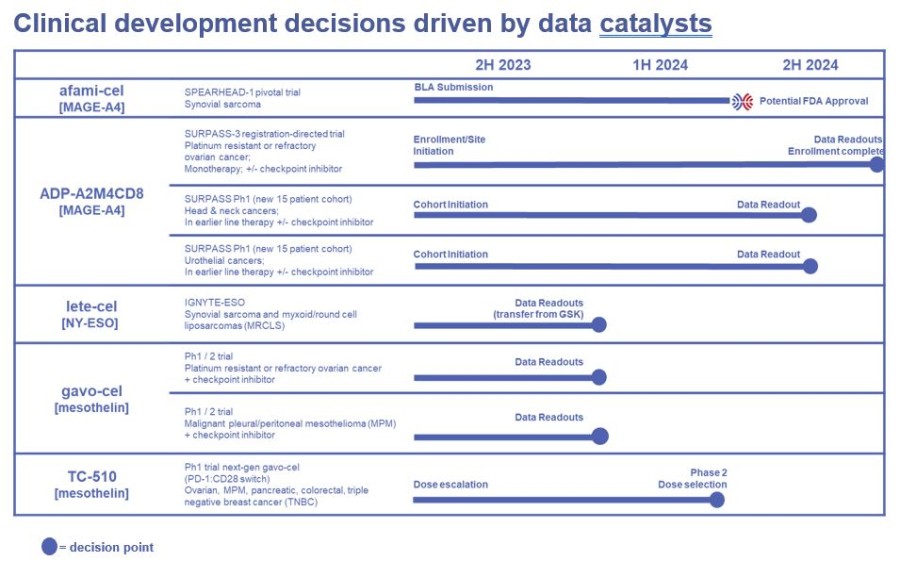
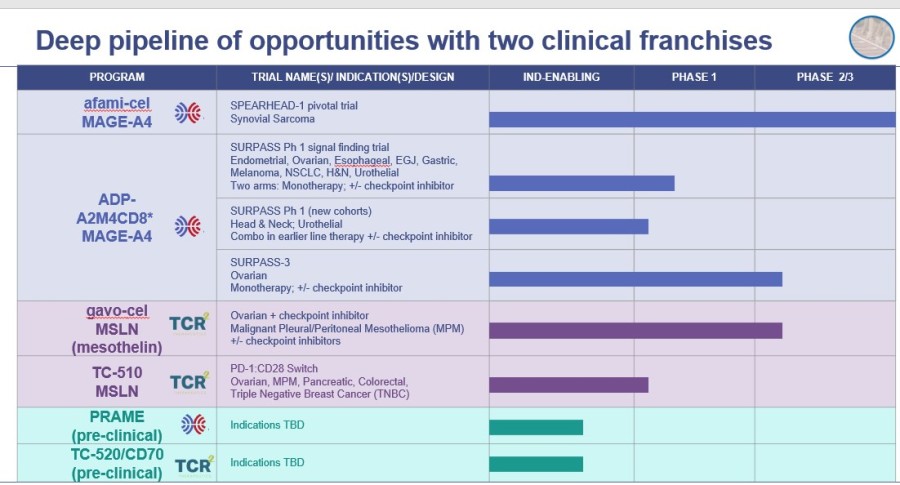
Tecelra® launch on track with 9 Authorized Treatment Centers available to initiate patient treatment journey, and the first patient apheresed in Q3; expect first commercial revenues in Q4 and the number of treated patients to accelerate throughout 2025 Lete-cel IGNYTE-ESO pivotal trial primary analysis reports 42% overall response rate in synovial sarcoma and myxoid/round cell liposarcoma (MRCLS); full data at CTOS... Read More



![Adaptimmune Therapeutics Reports Positive Data with Lete-cel[1] from an Interim Analysis of the Pivotal IGNYTE-ESO Trial for People with Synovial Sarcoma or MRCLS HealthStocksHub](/images/news/feeds/nwf/2023/ADAP185659_beb2fe8354602a65_001full.jpg-6540ecd842c4f-900px.jpg)