Philadelphia, Pennsylvania and Oxford, United Kingdom--(Newsfile Corp. - October 23, 2023) - Adaptimmune Therapeutics plc (Nasdaq: ADAP), a leader in cell therapy to treat cancer, presented clinical and translational data from its Phase 1 SURPASS clinical trial (NCT04044859) investigating the next-generation engineered T-cell therapy ADP-A2M4CD8 at the Annual European Society for Medical Oncology (ESMO) Congress today. The oral presentation was presented by Dr. Victor Moreno of START Madrid-FJD, Fundación Jiménez Díaz Hospital, in the proffered paper session - investigational monotherapy.
Dr. Victor Moreno, Director of Clinical Research at START Madrid-FJD, Fundación Jiménez Díaz Hospital, clinical investigator in SURPASS trial and presenter at ESMO: "The findings from the SURPASS study represent a groundbreaking milestone in the field of cell therapy for solid tumors. For the first time, the utilization of modified T-cells targeting MAGE-A4 has demonstrated remarkable efficacy in generating substantial responses across a spectrum of diseases, including head & neck, urothelial, and ovarian cancers. These results give us confidence that the field of cell therapy for solid tumors will continue to advance in the forthcoming years."
Dr. Elliot Norry, Chief Medical Officer at Adaptimmune: "Advanced-stage solid tumors remain a tremendous challenge to manage, as the current standard of care treatments often have limited efficacy and can be a tremendous burden on patients, healthcare providers, and caregivers. We are pleased to see promising responses and safety results with ADP-A2M4CD8 in people with advanced tumors who have generally received extensive prior therapies. Based on clear efficacy signals thus far in the Phase 1 SURPASS trial, we are eager to now focus on the head & neck and bladder cancer cohorts in earlier lines of treatment as well as the Phase 2 SURPASS-3 ovarian cancer trial."
Positive data from the Phase 1 SURPASS trial (ESMO 2023)
Clinical data demonstrate efficacy signals supporting further development in ovarian, urothelial and head & neck cancers. ADP-A2M4CD8 continues to show acceptable benefit to risk profile for people with a broad range of MAGE-A4 expressing unresectable or metastatic tumors, including in people receiving nivolumab combination therapy.
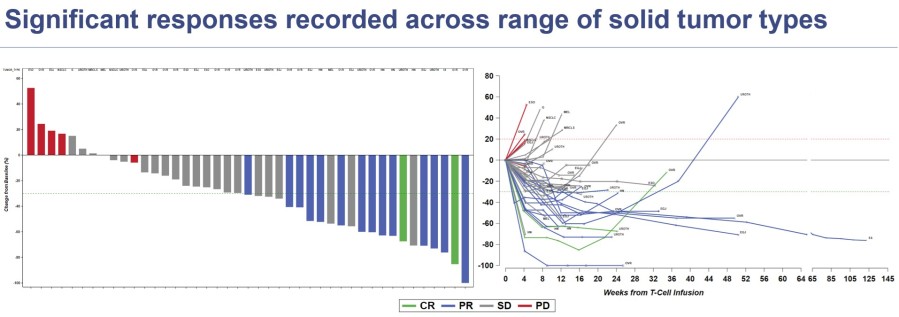 CR=complete response; PR=partial response; SD=stable disease; PD=progressive disease; ESMO 2023 data cut off August 14, 2023
CR=complete response; PR=partial response; SD=stable disease; PD=progressive disease; ESMO 2023 data cut off August 14, 2023
In the 26 people in the monotherapy arm with ovarian, urothelial, and head & neck cancers, there were 13 clinical responses, resulting in a 50% response rate. Among people with these indications who received 3 or fewer prior lines of systemic therapy, there was a 75% (9/12) response rate supporting future clinical development in earlier line treatment settings.
Early data from the nivolumab combination arm is still evolving. At the time of the data cut, there was 1 confirmed response. Subsequent to the data cut, there has been one additional confirmed response in the combination arm.
ADP-A2M4CD8 continues to show an acceptable benefit-to-risk profile among people with a broad range of MAGE-A4 expressing unresectable or metastatic tumors, including in those receiving nivolumab combination therapy. Cytokine release syndrome (CRS) was tolerable, and manageable with tocilizumab and corticosteroids when required.
Translational analyses performed on tumor biopsy and serum samples demonstrated that clinical responses are associated with strong evidence of ADP-A2M4CD8 tumor infiltration, broad immune engagement, and anti-MAGE-A4 tumor activity.
About the SURPASS Family of Trials
Phase 1 SURPASS trial (NCT04044859): ADP-A2M4CD8, a next-generation engineered T-cell therapy targeting the cancer testis antigen MAGE-A4, is being investigated for the treatment of advanced or unresectable solid tumors in the Phase 1 SURPASS clinical trial (NCT04044859). Enrollment is now focused on people with head & neck or urothelial cancers, in earlier lines of treatment and in combination with checkpoint inhibitors. This will facilitate further understanding of the early promising signals in these tumor types and enable decisions regarding later phase trials. Patients will receive a dose of 1 to 10 billion ADP-A2M4CD8 engineered T-cells following the lymphodepletion regimen of fludarabine (30 mg/m^2/day for 4 days) and cyclophosphamide (600 mg/m^2/day for 3 days). Key eligibility criteria include diagnosis of advanced head & neck or urothelial cancers; HLA-A*02 and MAGE-A4 positive tumor biopsies; ECOG performance status of 0 or 1; Aged ≥18 years and ≤75 years; Measurable disease per RECIST v1.1 prior to lymphodepletion.
SURPASS-3 trial (NCT05601752/ (GOG-3084): ADP-A2M4CD8 received FDA RMAT designation in 2022 for the treatment of patients with platinum resistant ovarian cancer and is currently enrolling.
This is a global Phase 2 clinical trial designed to evaluate ADP-A2M4CD8 TCR T-cell therapy alone or in combination with nivolumab in patients with recurrent or metastatic platinum resistant ovarian cancer (PROC). The SURPASS-3 trial will treat up to 66 patients randomized to receive ADP-A2M4CD8 either as monotherapy or in combination with nivolumab (checkpoint inhibitor) combination arms. Patients will receive a dose of 1 to 10 billion ADP-A2M4CD8 engineered T-cells following the lymphodepletion regimen of fludarabine (30 mg/m^2/day for 4 days) and cyclophosphamide (600 mg/m^2/day for 3 days). Patients enrolled in the combination arm will also receive nivolumab 480 mg every 4 weeks, starting at week 4 post engineered T-cell infusion. Key eligibility criteria include diagnosis of platinum-resistant ovarian carcinoma; HLA-A*02 and MAGE-A4 positive tumor biopsies; ECOG performance status of 0 or 1; Aged ≥18 years and ≤75 years; Measurable disease per RECIST v1.1 prior to lymphodepletion.
The primary endpoint is overall response (ORR) rate by RECIST v1.1 by independent review. Safety endpoints will be reviewed by an Independent Data Safety Monitoring Board. The trial is currently underway in collaboration with The GOG Foundation, Inc. (GOG) in the United States, with additional sites initiating in Canada, the United Kingdom, and the EU.
About Ovarian Cancer
Ovarian cancer is a group of diseases that originate in female ovaries, or in the related areas of the fallopian tubes and the peritoneum. In the U.S. each year, there are approximately 20,000 new cases, with more than 50% women diagnosed with metastatic disease, and more than 13,000 deaths.1 Platinum chemotherapy is standard of care for patients but about 70% of advanced patients have a recurrence after standard treatment.2,3,4 Many of these patients become resistant to platinum-based treatments (approximately 18,000 cases in 2023).5 Those with platinum-resistant ovarian cancer have limited options.
1.NCI SEER 2. NCCN guidelines 3. Mcquire et al. 1996 Jan 4;334(1):1-6 4. Ushijima J Oncol. 2010; 2010: 497429
5. Decision Resources Group DRG Ovarian Forecast Aug 2023.
About Head & Neck Cancer
Head & neck cancer is categorized as any cancer that occurs in the epithelium of the paranasal sinuses, nasopharynx, oropharynx, oral cavity, hypopharynx or larynx. 68,000 people are diagnosed with this cancer in the US per year.1 These cancers are more than twice as common among men as they are among women and more often diagnosed in people over age 50.1 In the US, the five-year survival across all those diagnosed with head & neck cancers is approximately 61-68%, declining to less than 40% for people diagnosed with metastatic disease.2 Immunotherapies represent the main treatment option for those with recurrent or metastatic disease, but response rates remain low, at approximately 19% for checkpoint inhibitor monotherapy and 36% for checkpoint inhibitors in combination with chemotherapy with median progression-free survival of 3-to-5 months and median overall survival of 13 to 15 months.3 People with later-line disease have limited effective options, median progression-free survival with standard care treatment is less than 4 months and median overall survival is less than 8 months.4,5,6
1. www.cancer.gov/types/head-and-neck/head-neck-fact-sheet, data as of 2021. Visited 10.12.2023. 2. National Cancer Institute Surveillance, and End Results Program (SEER) Program: Cancer Stat Facts: https://seer.cancer.gov/statfacts/. 3. Pembrolizumab/Keytruda Prescribing Information 4. Vermorken et al. 2008. Cancer. 112(12): 2710-9. 5.Knoedler et al. 2013. Oncology. 84:284-289. 6. Peron et. al. Anticancer Drugs. 2012. 23(9):996-1001.
About Urothelial Cancer/Bladder Cancers
Urothelial cancer is cancer that begins in the urothelial cells, which line the urethra, bladder, ureters, renal pelvis, and some other organs1. In the U.S. there are an estimated 82,000 new cases of bladder cancer and about 17,000 deaths each year. In the US, while 77% of people are alive 5 years after diagnosis, treatment options are suboptimal for people with advanced/metastatic disease.2 Only about half of the people treated with standard of care first-line cisplatin-based chemotherapy respond and people progress at a median of approximately 8 months.3,4 People ineligible for platinum chemotherapy may be treated with alternative regimens, with response rates of 41% -68%.5,6,7,8 People receiving checkpoint inhibitors after progressing on platinum have a response rate of approximately 20%9,10 and, for certain checkpoint inhibitors, median progression-free survival of approximately 2 months and median overall survival of 10 months.9
1. www.cancer.gov/types/bladder. Visited 10.12.23. 2. www.cancer.org/cancer/types/bladder-cancer/ about/key-statistics.html. 2. National Cancer Institute Surveillance, and End Results Program (SEER) Program: Cancer Stat Facts: https://seer.cancer.gov/statfacts/. 3. von der Maase et al. 2000. J Clin Oncol. 18(17):3068-77. 4. von der Maase et al. 2005. J Clin Oncol. 21:4602-4608. 5. De Santis et al. 2012. J Clin Oncol. 30(2):191-199. 6 Balar et al. 2017. Lancet. 389: 67-76. 7. Vuky et al. 2020. J Clin Oncol.. 38(23): 2658-2666. 8. Padcev Prescribing Information 9. Pembrolizumab/Keytruda Prescribing Information. 10. Nivolumab/Opdivo Prescribing Information.
About Adaptimmune
Adaptimmune is a clinical-stage biopharmaceutical company focused on designing, developing, and delivering cell therapies to transform the lives of people with cancer. The Company's unique engineered T-cell receptor (TCR) platform enables the engineering of T-cells to target and destroy cancers across multiple solid tumor types.
Forward-Looking Statements
This release contains "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995 (PSLRA). These forward-looking statements involve certain risks and uncertainties. Such risks and uncertainties could cause our actual results to differ materially from those indicated by such forward-looking statements, and include, without limitation: the success, cost and timing of our product development activities and clinical trials and our ability to successfully advance our TCR therapeutic candidates through the regulatory and commercialization processes. For a further description of the risks and uncertainties that could cause our actual results to differ materially from those expressed in these forward-looking statements, as well as risks relating to our business in general, we refer you to our Annual Report on Form 10-K filed with the Securities and Exchange Commission for the year ended December 31, 2022, our Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and other filings with the Securities and Exchange Commission. The forward-looking statements contained in this press release speak only as of the date the statements were made and we do not undertake any obligation to update such forward-looking statements to reflect subsequent events or circumstances.
Adaptimmune Contacts
Investor Relations
Juli P. Miller, Ph.D. - VP, Corporate Affairs and Investor Relations
T : +1 215 825 9310
M : +1 215 460 8920
This email address is being protected from spambots. You need JavaScript enabled to view it.
Media Relations
Dana Lynch, Senior Director of Corporate Communications
M: +1 267 990 1217
This email address is being protected from spambots. You need JavaScript enabled to view it.
| Last Trade: | US$0.67 |
| Daily Change: | -0.0028 -0.41 |
| Daily Volume: | 4,162,883 |
| Market Cap: | US$171.860M |
November 13, 2024 | |

ClearPoint Neuro is a global therapy-enabling platform company providing stereotactic navigation and delivery to the brain. Applications of our ClearPoint Neuro Navigation System include electrode lead placement, placement of catheters, and biopsy. The platform has FDA clearance and is...
CLICK TO LEARN MORE
Amneal Pharmaceuticals is a fully-integrated essential medicines company. We make healthy possible through the development, manufacturing, and distribution of generic and specialty pharmaceuticals. The Company has a diverse portfolio of over 250 products in its Generics segment and is expanding across...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB