Philadelphia, Pennsylvania and Oxford, United Kingdom--(Newsfile Corp. - August 9, 2023) - Adaptimmune Therapeutics plc (NASDAQ: ADAP), a leader in cell therapy to treat cancer, today reported financial results for the second quarter ended June 30, 2023 and provided a business update.
Adrian Rawcliffe, Adaptimmune's Chief Executive Officer: "The BLA submission process for afami-cel is going well with alignment with the Agency on key de-risking items. We have completed most of the wet work and we are now focused on writing and publishing the remaining sections to complete Part 3 of this submission for approval of the first engineered T-cell therapy for a solid tumor indication. We have completed the combination with TCR2 and added pipeline assets, technologies, and increased our total liquidity by approximately $85m. The transition of lete-cel back from GSK is also progressing well. We have set ourselves up to make data-driven portfolio decisions to bring medicines to market that we have high conviction can make a real difference for people with cancer."
Pipeline update and overview of near- and mid-term catalysts to make rigorous data-driven investment decisions
Adaptimmune's lead clinical franchises utilize engineered T-cell therapies targeting MAGE-A4, NY-ESO (in process of transitioning from GSK), and mesothelin, which are expressed on a broad range of solid tumors. Use of these cell therapies is supported by compelling clinical data including results in late-stage synovial sarcoma which will form the basis of the Company's first BLA submission. The Company has an enhanced "next-gen toolbox" and preclinical pipeline including PRAME and CD70 programs.
The following figure provides an overview of pipeline data catalysts that will be used to make data-driven investment decisions. . Note, this figure was previously provided in the Adaptimmune corporate deck and a press release issued on June 1st to announce completion of the strategic combination with TCR2.
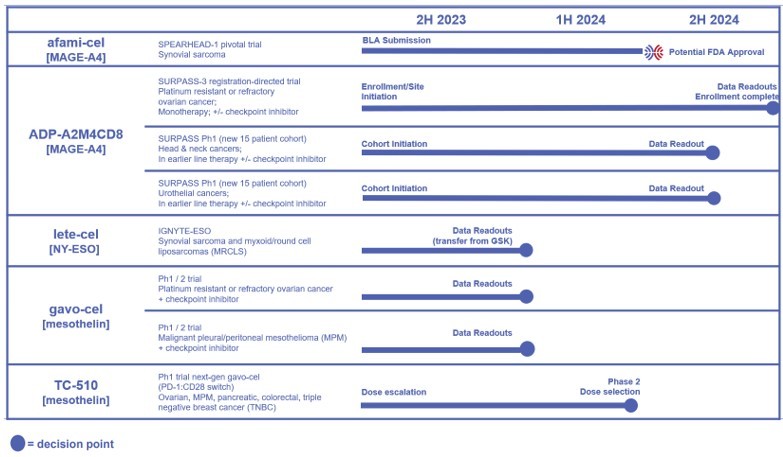
Afami-cel targeting MAGE-A4
Lete-cel targeting NY-ESO (in process of transitioning from GSK): could offer another potential commercial treatment for people with sarcoma
ADP-A2M4CD8: next-generation therapy targeting MAGE-A4
Gavo-cel targeting mesothelin
TC-510: next-generation therapy targeting mesothelin
Preclinical programs targeting PRAME and CD70 (not shown in figure)
Afami-cel: Adaptimmune plans to complete BLA submission in Q4 2023
In late 2022, Adaptimmune completed submission of the preclinical module (Part 1) of the BLA. In Q1 2023, the Company completed submission of the clinical module (Part 2). The Company is currently in the process of completing the final CMC module (Part 3).
Recently, several critical milestones have been completed:
This BLA is supported by data from Cohort 1 of the pivotal trial SPEARHEAD-1, which met its primary endpoint for efficacy. The Company has Regenerative Medicine Advanced Therapy (RMAT) designation from the FDA for afami-cel for the treatment of synovial sarcoma.
Afami-cel data presentation at ASCO 2023
Data from SPEARHEAD-1 was presented in a poster at the American Society of Clinical Oncology (ASCO) Annual Meeting, which is available HERE and summarized below.
Corporate news
Financial Results for the three and six months ended June 30, 2023
Financial Guidance
The Company believes that its existing cash, cash equivalents and marketable securities, together with the additional payments under the Strategic Collaboration and License Agreement with Genentech and payments under the Termination and Transfer Agreement with GSK, will fund the Company's current operations into early 2026, as further detailed in the Company's Quarterly Report on Form 10-Q for the second quarter ended June 30, 2023, to be filed with the Securities and Exchange Commission following this earnings release.
Webcast Information
The Company will host a live webcast to provide additional details at 8:00 a.m. EDT (1:00 p.m. BST) today, August 9, 2023. A live webcast of the conference call and replay can be accessed at https://www.gowebcasting.com/12658. Call in information is as follows: 1-800-806-54874 (US or Canada) or +1 (416)-340-2217 participant passcode 4991002# (International and additional options available HERE). Callers should dial in 5-10 minutes prior to the scheduled start time and simply ask to join the Adaptimmune call.
About Adaptimmune
Adaptimmune is a clinical-stage biopharmaceutical company focused on the development of novel cancer immunotherapy products for people with cancer. The Company's cell therapy products have shown clinical responses in multiple solid tumor indications. Our unique T-cell platforms enable us to identify cancer targets, find and develop cell therapy candidates active against those targets, and produce therapeutic candidates for administration to patients. Adaptimmune's cell therapy products include T-cells with genetically engineered T-cell receptors ("TCR T-cells"), TCR Fusion Construct T cells (TRuC-T cells), and HLA-independent TCRs ("HiTs").
Forward-Looking Statements
This release contains "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995 (PSLRA). These forward-looking statements involve certain risks and uncertainties. Such risks and uncertainties could cause our actual results to differ materially from those indicated by such forward-looking statements, and include, without limitation: the success, cost and timing of our product development activities and clinical trials and our ability to successfully advance our TCR therapeutic candidates through the regulatory and commercialization processes. For a further description of the risks and uncertainties that could cause our actual results to differ materially from those expressed in these forward-looking statements, as well as risks relating to our business in general, we refer you to our Annual Report on Form 10-K for the year ended December 31, 2022 filed with the Securities and Exchange Commission (SEC) on March 6, 2023, our Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and other filings with the SEC. The forward-looking statements contained in this press release speak only as of the date the statements were made and we do not undertake any obligation to update such forward-looking statements to reflect subsequent events or circumstances.
Total Liquidity (a non-GAAP financial measure)
Total Liquidity (a non-GAAP financial measure) is the total of cash and cash equivalents and marketable securities (available-for-sale debt securities). Each of these components appears separately in the condensed consolidated balance sheet. The U.S. GAAP financial measure most directly comparable to Total Liquidity is cash and cash equivalents as reported in the condensed consolidated financial statements, which reconciles to Total Liquidity as follows (in thousands):
| June 30, | December 31, | |||||
| 2023 | 2022 | |||||
| Cash and cash equivalents | $ | 76,969 | $ | 108,033 | ||
| Marketable securities - available-for-sale debt securities | 127,738 | 96,572 | ||||
| Total Liquidity | $ | 204,707 | $ | 204,605 | ||
The Company believes that the presentation of Total Liquidity provides useful information to investors because management reviews Total Liquidity as part of its assessment of overall solvency and liquidity, financial flexibility, capital position and leverage.
Condensed Consolidated Statement of Operations
(unaudited, in thousands, except per share data)
| Three months ended | Six months ended | |||||||||||
| June 30, | June 30, | |||||||||||
| 2023 | 2022 | 2023 | 2022 | |||||||||
| Revenue | $ | 5,130 | $ | 5,538 | $ | 52,731 | $ | 9,113 | ||||
| Operating expenses | ||||||||||||
| Research and development | (29,965) | (34,740) | (55,513) | (71,492) | ||||||||
| General and administrative | (20,073) | (14,550) | (40,470) | (31,354) | ||||||||
| Total operating expenses | (50,038) | (49,290) | (95,983) | (102,846) | ||||||||
| Operating loss | (44,908) | (43,752) | (43,252) | (93,733) | ||||||||
| Interest income | 1,543 | 357 | 2,219 | 695 | ||||||||
| Gain on bargain purchase | 22,155 | - | 22,155 | - | ||||||||
| Other income (expense), net | 501 | (655) | (170) | (643) | ||||||||
| Loss before income tax expense | (20,709) | (44,050) | (19,048) | (93,681) | ||||||||
| Income tax expense | (680) | (470) | (1,305) | (1,104) | ||||||||
| Net loss attributable to ordinary shareholders | $ | (21,389) | $ | (44,520) | $ | (20,353) | $ | (94,785) | ||||
| | ||||||||||||
| Net loss per ordinary share | ||||||||||||
| Basic and diluted | $ | (0.02) | $ | (0.05) | $ | (0.02) | $ | (0.10) | ||||
| | ||||||||||||
| | ||||||||||||
| Weighted average shares outstanding: | ||||||||||||
| Basic and diluted | 1,108,166,960 | 962,794,072 | 1,050,071,434 | 951,474,546 | ||||||||
| | ||||||||||||
Condensed Consolidated Balance Sheets
(unaudited, in thousands, except share data)
| June 30, | December 31, | |||||
| 2023 | 2022 | |||||
| Assets | ||||||
| Current assets | ||||||
| Cash and cash equivalents | $ | 76,969 | $ | 108,033 | ||
| Marketable securities - available-for-sale debt securities | 127,738 | 96,572 | ||||
| Accounts receivable, net of allowance for expected credit losses of $0 and $0 | 2,970 | 7,435 | ||||
| Other current assets and prepaid expenses | 54,094 | 43,330 | ||||
| Total current assets | 261,771 | 255,370 | ||||
| | ||||||
| Restricted cash | 3,231 | 1,569 | ||||
| Operating lease right-of-use assets, net of accumulated amortization of $11,258 and $9,470 | 22,027 | 18,019 | ||||
| Property, plant and equipment, net of accumulated depreciation of $40,635 and $38,588 | 55,492 | 53,516 | ||||
| Intangible assets, net of accumulated amortization of $5,003 and $4,676 | 463 | 442 | ||||
| Total assets | $ | 342,984 | $ | 328,916 | ||
| | ||||||
| Liabilities and stockholders' equity | ||||||
| Current liabilities | ||||||
| Accounts payable | $ | 14,713 | $ | 4,753 | ||
| Operating lease liabilities, current | 4,752 | 2,728 | ||||
| Accrued expenses and other current liabilities | 25,242 | 31,215 | ||||
| Restructuring provision | - | 2,285 | ||||
| Deferred revenue, current | 31,418 | 23,520 | ||||
| Total current liabilities | 76,125 | 64,501 | ||||
| | ||||||
| Operating lease liabilities, non-current | 21,590 | 20,349 | ||||
| Deferred revenue, non-current | 117,257 | 160,892 | ||||
| Other liabilities, non-current | 1,361 | 1,296 | ||||
| Total liabilities | 216,333 | 247,038 | ||||
| | ||||||
| Stockholders' equity | ||||||
| Common stock - Ordinary shares par value £0.001, 1,702,760,280 authorized and 1,351,828,044 issued and outstanding (2022: 1,282,773,750 authorized and 987,109,890 issued and outstanding) | 1,851 | 1,399 | ||||
| Additional paid in capital | 1,057,547 | 990,656 | ||||
| Accumulated other comprehensive loss | (3,092) | (875) | ||||
| Accumulated deficit | (929,655) | (909,302) | ||||
| Total stockholders' equity | 126,651 | 81,878 | ||||
| | ||||||
| Total liabilities and stockholders' equity | $ | 342,984 | $ | 328,916 | ||
Condensed Consolidated Cash Flow Statement
(unaudited, in thousands)
| Six months ended | ||||||
| June 30, | ||||||
| 2023 | 2022 | |||||
| Cash flows from operating activities | ||||||
| Net loss | $ | (20,353) | $ | (94,785) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||
| Depreciation | 3,824 | 2,728 | ||||
| Amortization | 253 | 419 | ||||
| Gain on bargain purchase | (22,155) | - | ||||
| Share-based compensation expense | 5,513 | 10,631 | ||||
| Unrealized foreign exchange losses/(gains) | 377 | (108) | ||||
| (Accretion)/amortization on available-for-sale debt securities | (633) | 1,636 | ||||
| Other | 663 | 585 | ||||
| Changes in operating assets and liabilities: | ||||||
| Decrease/(increase) in receivables and other operating assets | 1,971 | (22,898) | ||||
| (Decrease)/increase in payables and other current liabilities | (8,801) | 12,898 | ||||
| Decrease in deferred revenue | (41,704) | (6,758) | ||||
| Net cash used in operating activities | (81,045) | (95,652) | ||||
| Cash flows from investing activities | ||||||
| Acquisition of property, plant and equipment | (3,565) | (16,074) | ||||
| Acquisition of intangible assets | (199) | - | ||||
| Acquired upon acquisition of TCR2 Therapeutics Inc. | 45,264 | - | ||||
| Maturity or redemption of marketable securities | 76,119 | 97,605 | ||||
| Investment in marketable securities | (67,121) | (42,197) | ||||
| Other | 537 | - | ||||
| Net cash provided by investing activities | 51,035 | 39,334 | ||||
| Cash flows from financing activities | ||||||
| Proceeds from issuance of common stock from offerings, net of commissions and issuance costs | 188 | 9,976 | ||||
| Proceeds from exercise of stock options | 22 | 36 | ||||
| Net cash provided by financing activities | 210 | 10,012 | ||||
| Effect of currency exchange rate changes on cash, cash equivalents and restricted cash | 398 | (5,836) | ||||
| Net decrease in cash, cash equivalents and restricted cash | (29,402) | (52,142) | ||||
| Cash, cash equivalents and restricted cash at start of period | 109,602 | 151,666 | ||||
| Cash, cash equivalents and restricted cash at end of period | $ | 80,200 | $ | 99,524 | ||
Adaptimmune Contact
Investor Relations
Juli P. Miller, Ph.D. - VP, Corporate Affairs and Investor Relations
T: +1 215 825 9310
M: +1 215 460 8920
This email address is being protected from spambots. You need JavaScript enabled to view it.
Media Relations
Dana Lynch, Senior Director of Corporate Communications
M: +1 267 990 1217
This email address is being protected from spambots. You need JavaScript enabled to view it.
1 Total liquidity is a non-GAAP financial measure, which is explained and reconciled to the most directly comparable financial measures prepared in accordance with GAAP below.
| Last Trade: | US$0.67 |
| Daily Change: | -0.0028 -0.41 |
| Daily Volume: | 4,162,883 |
| Market Cap: | US$171.860M |
November 13, 2024 | |

Cue Biopharma is developing the first-ever class of therapeutics for the treatment of cancer that mimic the natural signals, or “Cues”, of the immune system. This novel class of injectable biologics selectively engages and modulates tumor-specific T cells directly within the patient’s body to transform...
CLICK TO LEARN MORE
Astria Therapeutics is a biopharmaceutical company, and our mission is to bring life-changing therapies to patients and families affected by rare and niche allergic and immunological diseases. Our lead program, STAR-0215, is a monoclonal antibody inhibitor of plasma kallikrein in clinical development...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB