Philadelphia, Pennsylvania, Oxford, United Kingdom, and Cambridge, Massachusetts--(Newsfile Corp. - March 6, 2023) - Adaptimmune Therapeutics plc (NASDAQ: ADAP) and TCR² Therapeutics Inc. (NASDAQ: TCRR), today announced entry into a definitive agreement under which Adaptimmune will combine with TCR² in an all-stock transaction to create a preeminent cell therapy company focused on treating solid tumors. The combination provides extensive benefits for clinical development and product delivery supported by complementary technology platforms. As a result, and following the closing of the transaction, it is anticipated that the combined company's cash runway will extend into 2026.
The lead clinical franchises for the combined company utilize engineered T-cell therapies targeting MAGE-A4 and mesothelin. These targets are expressed on a broad range of solid tumors and are supported by compelling early- and late-stage clinical data. The combined company also has a preclinical pipeline of additional target opportunities with development initially focused on PRAME and CD70.
Adrian Rawcliffe, Adaptimmune's Chief Executive Officer: "This strategic combination takes two technologically and culturally aligned companies at the forefront of their fields and combines them to create a preeminent cell therapy company for solid tumors. The combined company will drive forward its pipeline of cell therapies aimed at treating multiple cancers with high unmet medical needs. This includes gaining approval for the first engineered TCR T-cell therapy for a solid tumor - afami-cel for the treatment of synovial sarcoma. With our cash runway anticipated to be extended into 2026 and covering multiple clinical catalysts in cancers with significant market potential, the combined company is well placed to develop cell therapies as a mainstream option for people with cancer."
Garry Menzel, Ph.D., President and Chief Executive Officer of TCR2 Therapeutics: "The strategic rationale for this combination and the operating benefits are highly compelling for both Adaptimmune and TCR² shareholders. The combination of our two companies not only sets the stage for near-term execution but also positions the new company for the longer-term. We jointly have an array of next-generation innovations that we will integrate to address the tumor micro-environment using both autologous and allogeneic approaches. Focus and specialization are critical in the cell therapy space and we believe the combined company has the technologies necessary to succeed. I am delighted that this combination provides a strong foundation to commercialize curative therapies for people with cancer."
Significant Solid Tumor Opportunity
Solid tumors represent approximately 90% of all cancers. The combined company's clinical programs targeting MAGE-A4 or mesothelin can address multiple solid tumor indications with the potential to treat >300,000 patients per year in the EU and US.
For patients with tumors potentially expressing MAGE-A4 and mesothelin, the combined company plans to screen for both targets to identify eligible patients.
In addition, the preclinical pipeline, including PRAME and CD70, could expand the addressable population.
Complementary Technology Platforms
The combined company will possess two clinically validated and complementary platforms in SPEAR and TRuC T-cells enabling engagement of both intracellular targets (with SPEAR) and extracellular targets (with TRuC), thus broadening the potential number of addressable cancers.
Adaptimmune's proprietary SPEAR T-cell technology is based on the affinity enhancement and engineering of T-cell receptors (TCRs) to target solid tumor-specific peptide: HLA complexes.
TCR2's proprietary TRuC T-cell technology uses an antibody-based binding domain fused to TCR subunits to reprogram an intact TCR complex to recognize tumor surface antigens.
Both technologies can be further leveraged in the combined company's allogeneic platform.
Highly Specialized Talent and Operational Benefits
The novelty, complexity, and rapid growth of the cell therapy field has highlighted the need for companies to develop specialized capabilities with a goal of delivering treatments that are both curative and mainstream.
To this end, over the last decade, the teams at Adaptimmune and TCR2 have been responsible for successfully advancing multiple programs from preclinical concept to late-stage products.
The combined company, located in key innovation hubs, will have a deep bench of cell therapy professionals, infrastructure, and end-to-end capabilities.
Value-Creating Catalysts (see Exhibit A for combined clinical pipeline)
Following closing of the transaction, the combined company is anticipated to have a cash runway into 2026 providing operational benefits and enables delivery of key catalysts, including:
2023
Products targeting MAGE-A4
Afami-cel
Completion of BLA submission for the treatment of synovial sarcoma. Anticipated mid-year.
Supported by compelling clinical data from the pivotal SPEARHEAD-1 trial with a response rate of 39% after a single dose and a duration of 12 months.
ADP-A2M4CD8 (next-generation product)
Expected full data readout from the monotherapy portion of the Phase 1 SURPASS trial in heavily pre-treated patients across a broad range of solid tumors.
Initiation of the Phase 2 SURPASS-3 trial in combination with nivolumab for platinum resistant ovarian cancer. This trial has the potential to become registrational.
Initiation of additional cohorts in the Phase 1 SURPASS trial in combination with pembrolizumab to treat patients in the first-line treatment setting for head & neck cancer and second-line setting for urothelial cancer.
ADP-A2M4CD8 has demonstrated a 52% response rate in the focus indications of ovarian, urothelial, and head & neck cancers, which improves to a 75% response rate in patients who received 3 or few prior lines of therapy.
Products targeting mesothelin
Gavo-cel
First readout from the Phase 2 portion of the gavo-cel clinical trial in platinum resistant or refractory ovarian cancer. Anticipated year-end.
Interim update, including key translational data, in patients with mesothelioma treated with gavo-cel in the Phase 2 clinical trial before the focus was narrowed to ovarian cancer. Anticipated mid-year.
Tumor regression has been observed in 93% of patients in the Phase 1 trial. The response rate was 29% in patients with ovarian cancer with a progression free survival of 5.8 months and overall survival of 8.1 months. The response rate in mesothelioma was 21% with a progression free survival of 5.9 months and overall survival of 11.2 months.
TC-510 (next-generation product)
Preclinical
2024
Products targeting MAGE-A4
Afami-cel
ADP-A2M4CD8 (next-generation product)
First readout from SURPASS-3 trial in ovarian cancer
First readout for head and neck cancer cohort in the Phase 1 SURPASS trial
First readout for urothelial cancer cohort in the Phase 1 SURPASS trial
Products targeting mesothelin
Gavo-cel and TC-510
Readout from gavo-cel Phase 2 trial in platinum resistant ovarian cancer
Readout from TC-510 Phase 1 trial and selection of dose to carry forward into additional late-phase trials
Preclinical
Transaction details for strategic combination
The merger agreement was unanimously approved by the boards of directors of both companies. TCR2 stockholders will receive 1.5117 Adaptimmune ADS for each TCR2 share.
Following the closing of the transaction, Adaptimmune shareholders will own approximately 75% of the combined company and TCR2 stockholders will own approximately 25% of the combined company.
Subject to shareholder approval and the subsequent closing of the transaction, the combined company is expected to continue to trade on the Nasdaq Stock Market under the symbol "ADAP". The combined company has a team of leading cell therapy experts led by Adrian Rawcliffe, the CEO of Adaptimmune. The Board of Directors, composed of three members from TCR2 and six continuing from Adaptimmune, is expected to be: David Mott (Chair); Andrew Allen, M.D., Ph.D.; Lawrence Alleva; Ali Behbahani, M.D.; John Furey; Priti Hegde, Ph.D.; Garry Menzel, Ph.D.; Adrian Rawcliffe, and Elliott Sigal, M.D., Ph.D. (who will step down on November 1, 2023 when Kristen Hege, M.D. joins the Board of Directors).
The transaction is currently expected to close in Q2 2023, subject to the receipt of approvals by Adaptimmune shareholders and TCR2 stockholders and satisfaction or waiver of other closing conditions.
Adaptimmune Full Year 2022 Financial Results
In a separate press release, Adaptimmune will announce its Q4 and full year 2022 financial results and business updates, which will be available on the "Investor Relations" section of the Adaptimmune website.
Advisors
TD Cowen is serving as financial advisor to Adaptimmune and Ropes & Gray LLP is serving as legal counsel to Adaptimmune. Piper Sandler is serving as financial advisor to TCR2 and Goodwin Procter LLP is serving as legal counsel to TCR2.
Forward-Looking Statements
This communication relates to the proposed transaction pursuant to the terms of the Agreement and Plan of Merger, dated March 05, 2023, by and among Adaptimmune Therapeutics plc ("Parent"), CM Merger Sub, Inc. ("Merger Sub"), and TCR² Therapeutics Inc. (the "Company"). This communication includes express or implied forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended (the "Exchange Act"), about the proposed transaction between the Company and Parent and the operations of the combined company that involve risks and uncertainties relating to future events and the future performance of Parent and the Company. Actual events or results may differ materially from these forward-looking statements. Words such as "will," "could," "would," "should," "expect," "plan," "anticipate," "intend," "believe," "estimate," "predict," "project," "potential," "continue," "future," "opportunity" "will likely result," "target," variations of such words, and similar expressions or negatives of these words are intended to identify such forward-looking statements, although not all forward-looking statements contain these identifying words. Examples of such forward-looking statements include, but are not limited to, express or implied statements regarding: the business combination and related matters, including, but not limited to, satisfaction of closing conditions to the proposed transaction, prospective performance and opportunities with respect to Parent or the Company, post-closing operations and the outlook for the companies' businesses; Parent's, the Company's or the combined company's targets, plans, objectives or goals for future operations, including those related to Parent's and the Company's product candidates, research and development, product candidate introductions and product candidate approvals as well as cooperation in relation thereto; projections of or targets for revenues, costs, income (or loss), earnings per share, capital expenditures, dividends, capital structure, net financials and other financial measures; future economic performance, future actions and outcome of contingencies such as legal proceedings; and the assumptions underlying or relating to such statements. These statements are based on Parent's and the Company's current plans, estimates and projections. By their very nature, forward-looking statements involve inherent risks and uncertainties, both general and specific. A number of important factors, including those described in this communication, could cause actual results to differ materially from those contemplated in any forward-looking statements. Factors that may affect future results and may cause these forward-looking statements to be inaccurate include, without limitation: uncertainties as to the timing for completion of the proposed transaction; uncertainties as to the Company's and/or Parent's ability to obtain the approval of Parent's shareholders or the Company's stockholders required to consummate the proposed transaction; the possibility that competing offers will be made by third parties; the occurrence of events that may give rise to a right of one or both of Parent and the Company to terminate the merger agreement; the possibility that various closing conditions for the proposed transaction may not be satisfied or waived on a timely basis or at all, including the possibility that a governmental entity may prohibit, delay, or refuse to grant approval, if required, for the consummation of the proposed transaction (or only grant approval subject to adverse conditions or limitations); the difficulty of predicting the timing or outcome of consents or regulatory approvals or actions, if any; the possibility that the proposed transaction may not be completed in the time frame expected by Parent and the Company, or at all; the risk that Parent and Company may not realize the anticipated benefits of the proposed transaction in the time frame expected, or at all; the effects of the proposed transaction on relationships with Parent's or the Company's employees, business or collaboration partners or governmental entities; the ability to retain and hire key personnel; potential adverse reactions or changes to business relationships resulting from the announcement or completion of the proposed transaction; significant or unexpected costs, charges or expenses resulting from the proposed transaction; the potential impact of unforeseen liabilities, future capital expenditures, revenues, costs, expenses, earnings, synergies, economic performance, indebtedness, financial condition and losses on the future prospects, business and management strategies for the management, expansion and growth of the combined business after the consummation of the proposed transaction; potential negative effects related to this announcement or the consummation of the proposed transaction on the market price of Parent's American Depositary Shares or the Company's common stock and/or Parent's or the Company's operating or financial results; uncertainties as to the long-term value of Parent's American Depositary Shares (and the ordinary shares represented thereby), including the dilution caused by Parent's issuance of additional American Depositary Shares (and the ordinary shares represented thereby) in connection with the proposed transaction; unknown liabilities related to Parent or the Company; the nature, cost and outcome of any litigation and other legal proceedings involving Parent, the Company or their respective directors, including any legal proceedings related to the proposed transaction; risks related to global as well as local political and economic conditions, including interest rate and currency exchange rate fluctuations; potential delays or failures related to research and/or development of Parent's or the Company's programs or product candidates; risks related to any loss of Parent's or the Company's patents or other intellectual property rights; any interruptions of the supply chain for raw materials or manufacturing for Parent or the Company's product candidates, the nature, timing, cost and possible success and therapeutic applications of product candidates being developed by Parent, the Company and/or their respective collaborators or licensees; the extent to which the results from the research and development programs conducted by Parent, the Company, and/or their respective collaborators or licensees may be replicated in other studies and/or lead to advancement of product candidates to clinical trials, therapeutic applications, or regulatory approval; uncertainty of the utilization, market acceptance, and commercial success of Parent or the Company's product candidates, and the impact of studies (whether conducted by Parent, the Company or others and whether mandated or voluntary) on any of the foregoing; unexpected breaches or terminations with respect to Parent's or the Company's material contracts or arrangements; risks related to competition for Parent's or the Company's product candidates; Parent's or the Company's ability to successfully develop or commercialize Parent's or the Company's product candidates; Parent's, the Company's, and their collaborators' abilities to continue to conduct current and future developmental, preclinical and clinical programs; potential exposure to legal proceedings and investigations; risks related to changes in governmental laws and related interpretation thereof, including on reimbursement, intellectual property protection and regulatory controls on testing, approval, manufacturing, development or commercialization of any of Parent's or the Company's product candidates; unexpected increase in costs and expenses with respect to the potential transaction or Parent's or the Company's business or operations; and risks and uncertainties related to epidemics, pandemics or other public health crises and their impact on Parent's and the Company's respective businesses, operations, supply chain, patient enrollment and retention, preclinical and clinical trials, strategy, goals and anticipated milestones. While the foregoing list of factors presented here is considered representative, no list should be considered to be a complete statement of all potential risks and uncertainties. There can be no assurance that the proposed transaction or any other transaction described above will in fact be consummated in the manner described or at all. A more complete description of these and other material risks can be found in Parent's and the Company's respective filings with the U.S. Securities and Exchange Commission (the "SEC"), including each of their Annual Reports on Form 10-K for the year ended December 31, 2021, subsequent Quarterly Reports on Form 10-Q and other documents that may be filed from time to time with the SEC, as well as, the Registration Statement on Form S-4 which includes the joint proxy statement of Parent and the Company that also constitutes the prospectus of Parent, which joint proxy statement/prospectus will be mailed or otherwise disseminated to Parent's shareholders and the Company's stockholders when it becomes available. Parent and the Company also plan to file other relevant documents with the SEC regarding the proposed transaction. Any forward-looking statements speak only as of the date of this communication and are made based on the current beliefs and judgments of Parent's and the Company's management, and the reader is cautioned not to rely on any forward-looking statements made by Parent or the Company. Unless required by law, neither Parent nor the Company is under no duty and undertakes no obligation to update or revise any forward-looking statement after the distribution of this document, including without limitation any financial projection or guidance, whether as a result of new information, future events or otherwise.
No Offer or Solicitation
This communication is not intended to and shall not constitute an offer to subscribe for, buy or sell or the solicitation of an offer to subscribe for, buy or sell any securities, or a solicitation of any vote or approval, nor shall there be any sale of, or offer to sell or buy, securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. This communication is for informational purposes only. No offering of securities shall be made, except by means of a prospectus meeting the requirements of Section 10 of the U.S. Securities Act of 1933, as amended, and otherwise in accordance with applicable law.
Additional Information and Where to Find It
In connection with the proposed transaction, Parent and the Company expect to file with the SEC a Registration Statement on Form S-4. The Registration Statement on Form S-4 will include a document that serves as a prospectus of Parent and a joint proxy statement of Parent and the Company, and each party may also file other documents regarding the proposed transaction with the SEC. INVESTORS AND SECURITY HOLDERS ARE URGED TO READ CAREFULLY THE REGISTRATION STATEMENT ON FORM S-4, JOINT PROXY STATEMENT/PROSPECTUS AND OTHER RELEVANT DOCUMENTS FILED OR WILL BE FILED WITH THE SEC, AS WELL AS ANY AMENDMENTS OR SUPPLEMENTS THERETO AND ANY DOCUMENTS INCORPORATED BY REFERENCE THEREIN, IN THEIR ENTIRETY IF AND WHEN THEY BECOME AVAILABLE BECAUSE THEY CONTAIN OR WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTION, RELATED MATTERS AND THE PARTIES TO THE PROPOSED TRANSACTION.
You may obtain a free copy of the Registration Statement on Form S-4, joint proxy statement/prospectus and other relevant documents (if and when they become available) that are or will be filed with the SEC for free at the SEC's website at www.sec.gov. Copies of the documents filed with the SEC by the Company will be available free of charge on the Company's website at or by contacting the Company's Investor Relations Department at. Copies of the documents filed with the SEC by Parent will be available free of charge on Parent's website at [https://www.adaptimmune.com/investors-and-media/sec-filings] or by contacting Parent's Investor Relations Department at This email address is being protected from spambots. You need JavaScript enabled to view it..
Participants in the Solicitation
Parent, the Company and certain of their respective directors and executive officers and other members of management and employees may be deemed to be participants in the solicitation of proxies in respect of the proposed transaction. Information about the directors and executive officers of Parent, including a description of their direct or indirect interests, by security holdings or otherwise, is set forth in Parent's proxy statement for its 2022 Annual General Meeting, which was filed with the SEC on April 21, 2022, the Annual Report on Form 10-K for the year ended December 31, 2021 filed with the SEC on March 14, 2022, subsequent Quarterly Reports on Form 10-Q and other documents that may be filed from time to time with the SEC. Information about the directors and executive officers of the Company, including a description of their direct or indirect interests, by security holdings or otherwise, is set forth in the Company's proxy statement for its 2022 Annual Meeting of Stockholders, which was filed with the SEC on September 1, 2022, the Annual Report on Form 10-K for the year ended December 31, 2021 filed with the SEC on March 22, 2022, subsequent Quarterly Reports on Form 10-Q and other documents that may be filed from time to time with the SEC. Other information regarding the participants in the proxy solicitations and a description of their direct and indirect interests, by security holdings or otherwise, will be contained in the joint proxy statement/prospectus included in the Registration Statement on Form S-4 and other relevant materials to be filed with the SEC regarding the proposed transaction when such materials become available. Security holders, potential investors and other readers should read the joint proxy statement/prospectus, included in the Registration Statement on Form S-4 carefully when it becomes available before making any voting or investment decision. You may obtain free copies of these documents from Parent or the Company using the sources indicated above.
Webcast Information
A joint webcast will be held at 8:00 a.m. EST (1:00 p.m. GMT) on March 6, 2023. A live webcast of the conference call and replay can be accessed at https://api.newsfilecorp.com/redirect/e4WKKsxwna. Call in information is as follows: (800)-319-4610 (US or Canada) or +1 (416)-915-3239 (International and additional options available HERE). Callers should dial in 5-10 minutes prior to the scheduled start time and simply ask to join the Adaptimmune call.
Adaptimmune Contacts
Investor Relations
Juli P. Miller, Ph.D. - VP, Corporate Affairs and Investor Relations
This email address is being protected from spambots. You need JavaScript enabled to view it.
Media Relations
Dana Lynch, Senior Director of Corporate Communications
This email address is being protected from spambots. You need JavaScript enabled to view it.
TCR² Contacts
Investor Relations
Eric Sullivan
Chief Financial Officer
This email address is being protected from spambots. You need JavaScript enabled to view it.
Media Relations
Kathy Vincent
This email address is being protected from spambots. You need JavaScript enabled to view it.
Exhibit A - Combined Clinical Pipeline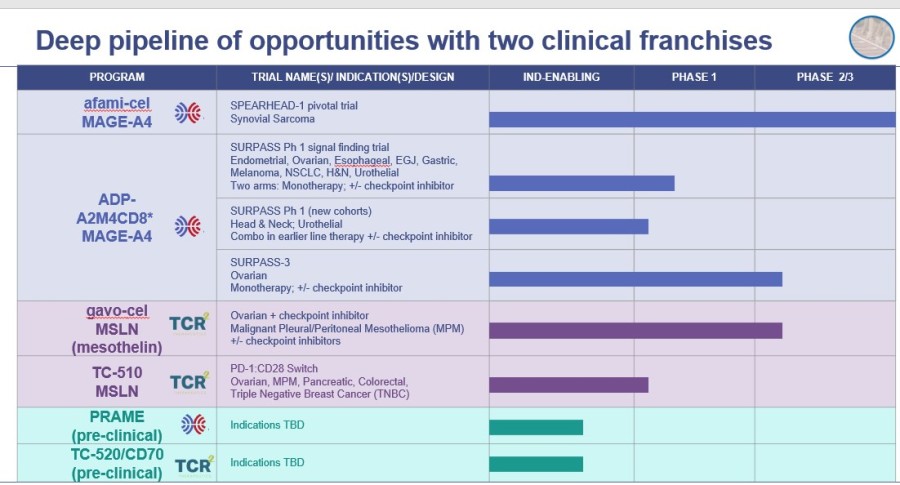
| Last Trade: | US$0.67 |
| Daily Change: | -0.0028 -0.41 |
| Daily Volume: | 4,162,883 |
| Market Cap: | US$171.860M |
November 13, 2024 | |

Astria Therapeutics is a biopharmaceutical company, and our mission is to bring life-changing therapies to patients and families affected by rare and niche allergic and immunological diseases. Our lead program, STAR-0215, is a monoclonal antibody inhibitor of plasma kallikrein in clinical development...
CLICK TO LEARN MORE
Amneal Pharmaceuticals is a fully-integrated essential medicines company. We make healthy possible through the development, manufacturing, and distribution of generic and specialty pharmaceuticals. The Company has a diverse portfolio of over 250 products in its Generics segment and is expanding across...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB