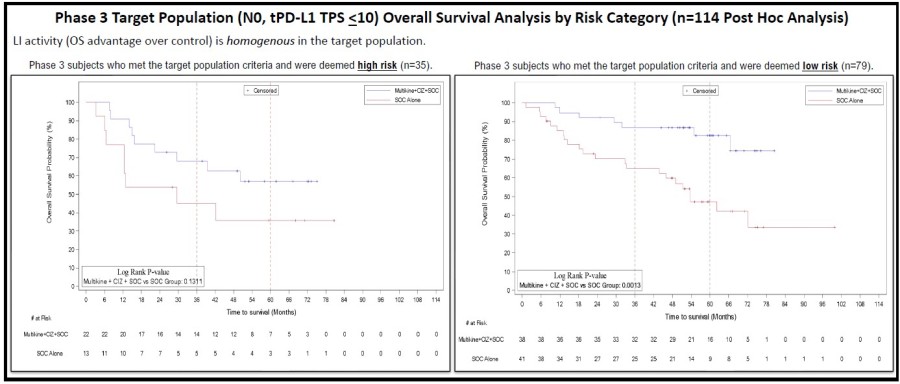
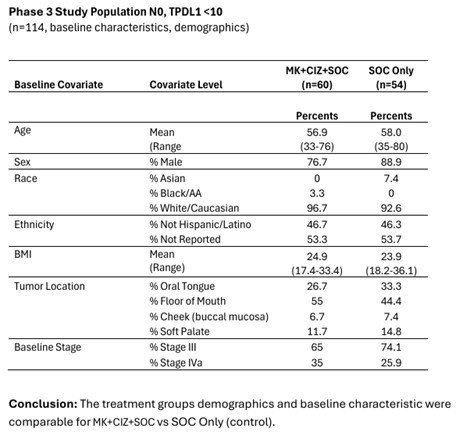
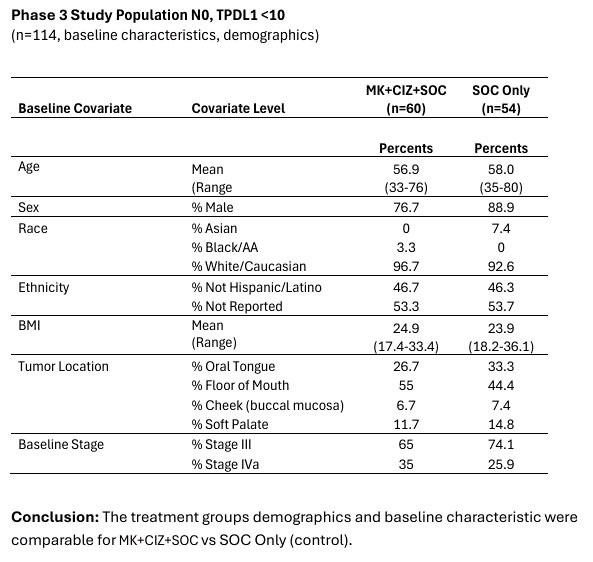
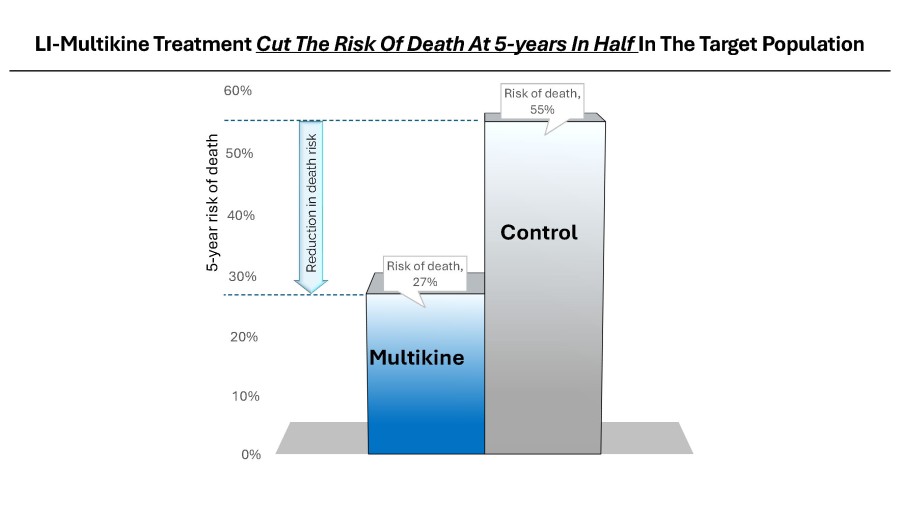
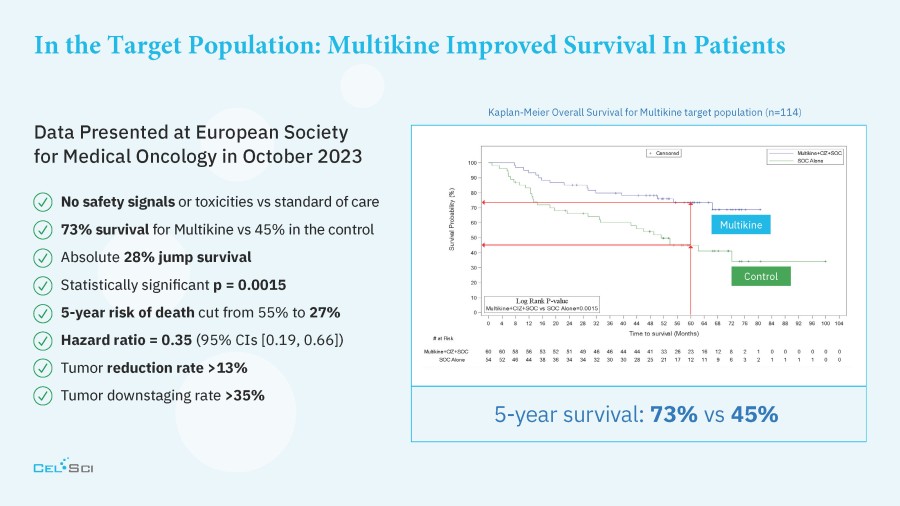
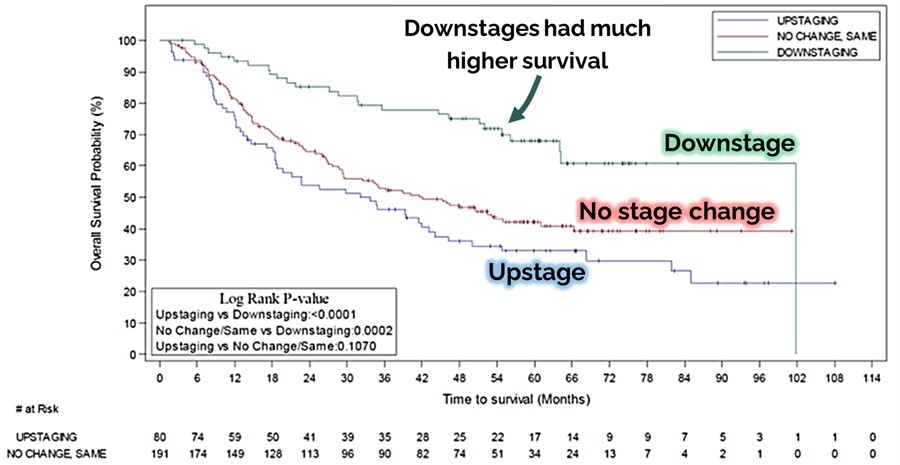
Multikine is the first cancer immunotherapy to show pre-surgical tumor regression in head and neck cancer in just 3 weeks - confirmed by pathology at surgery Factors supporting the go-ahead for the 212-person registration study in the target population include: Multikine led to significant rates of tumor regression Pre-surgical tumor regressions were confirmed at surgery and forecast survival benefit Target population is... Read More