VIENNA, Va. / Jun 18, 2024 / Business Wire / CEL-SCI Corporation (NYSE American: CVM) today announced the Company’s Chief Scientific Officer, Dr. Eyal Talor, delivered a presentation titled “Neoadjuvant Immunotherapy for Head and Neck Cancer: Low Tumor PD-L1 Expression - IT-MATTERS – RCT” at the International Drug Discovery Science & Technology (IDDST) 20th Annual Congress in Budapest, Hungary on Tuesday, June 18, 2024. Dr. Talor presented during the Cancers/Tumors session which he Chaired along with Dr. Elizabeth Tran of Purdue University.
Multikine* (Leukocyte Interleukin, Injection) is the first investigational pre-surgical cancer drug intended for use in newly diagnosed locally advanced resectable head and neck cancer. There is extensive affirmative safety and efficacy data from 750 patients who have been treated with Multikine. A randomized, controlled, Phase 3 trial (RCT) in head and neck cancer revealed that Multikine significantly increases overall survival in patients with low levels of tumor cell PD-L1 expression in contrast to checkpoint inhibitors (e.g. Keytruda, Opdivo) which show longer survival benefit in patients with a higher level of tumor cell PD-L1 expression. Tumor cell PD-L1, also known as Programmed Death-Ligand 1, is a protein that plays a crucial role in immune system regulation. It is the target pathway for immune checkpoint inhibitors, a major class of immune-oncology drugs which work by blocking the interaction between tumor cell PD-L1 and the PD-1 receptor on immune effector cells, thereby favoring immune evasion by the tumor.
“The survival benefit of Multikine we observed from previous data for the same study population is much higher than that which will be required to be successful in the confirmatory study,” stated Dr. Talor.
The presentation may be accessed on CEL-SCI’s website at the following: https://cel-sci.com/wp-content/uploads/2024/06/Scientific_Presentation_June-2024-Final.pdf
About CEL-SCI Corporation
CEL-SCI believes that boosting a patient’s immune system while it is still intact should provide the greatest possible impact on survival. Multikine is designed to help the immune system "target" the tumor at a time when the immune system is still relatively intact and thereby thought to be better able to mount an attack on the tumor.
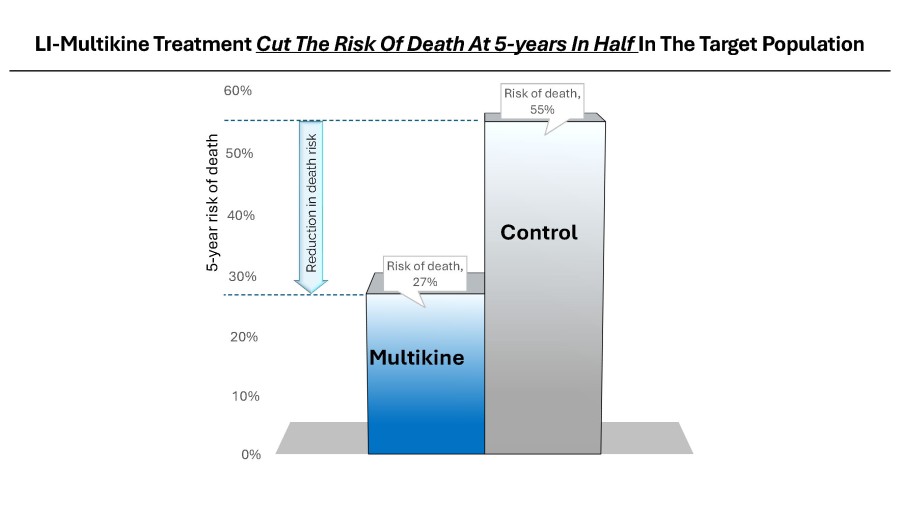
Multikine (Leukocyte Interleukin, Injection), a true first-line cancer therapy, has been dosed in over 750 patients and received Orphan Drug designation from the FDA for neoadjuvant therapy in patients with squamous cell carcinoma (cancer) of the head and neck. Multikine significantly extended life in its target patient population demonstrating a 73% survival rate with Multikine treatment regimen plus standard of Care (SOC) vs. only 45% for SOC without Multikine, at 5 years after treatment, Kaplan-Meier life table log rank p-value 0.0015. Based on this very strong data, the FDA concurred with CEL-SCI’s target patient selection criteria and gave the go-ahead to conduct a smaller, focused, confirmatory Registration Study of 212 patients. CEL-SCI will enroll newly diagnosed treatment naïve locally advanced primary SCC of the head and neck (oral cavity) cancer patients with no lymph node involvement (determined via PET scan) and with low PD-L1 tumor expression (determined via biopsy). Globally, there are approximately 100,000 patients meeting these diagnostic criteria annually.
The Company has operations in Vienna, Virginia, and near/in Baltimore, Maryland.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. When used in this press release, the words "intends," "believes," "anticipated," "plans" and "expects," and similar expressions, are intended to identify forward-looking statements. Such statements are subject to risks and uncertainties that could cause actual results to differ materially from those projected. Factors that could cause or contribute to such differences include an inability to duplicate the clinical results demonstrated in clinical studies, timely development of any potential products that can be shown to be safe and effective, receiving necessary regulatory approvals, difficulties in manufacturing any of the Company's potential products, inability to raise the necessary capital and the risk factors set forth from time to time in CEL-SCI's filings with the Securities and Exchange Commission, including but not limited to its report on Form 10-K for the year ended September 30, 2023. The Company undertakes no obligation to publicly release the result of any revision to these forward-looking statements which may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
* Multikine (Leukocyte Interleukin, Injection) is the trademark that CEL-SCI has registered for this investigational therapy. This proprietary name is subject to FDA review in connection with the Company's future anticipated regulatory submission for approval. Multikine has not been licensed or approved for sale, barter or exchange by the FDA or any other regulatory agency. Similarly, its safety or efficacy has not been established for any use.
| Last Trade: | US$0.62 |
| Daily Change: | -0.14 -18.09 |
| Daily Volume: | 1,419,416 |
| Market Cap: | US$39.560M |
December 12, 2024 November 07, 2024 September 10, 2024 | |

Compass Therapeutics is a clinical-stage, oncology-focused biopharmaceutical company developing proprietary antibody-based therapeutics to treat multiple human diseases. The company's scientific focus is on the relationship between angiogenesis, the immune system, and tumor growth...
CLICK TO LEARN MORE
C4 Therapeutics is pioneering a new class of small-molecule drugs that selectively destroy disease-causing proteins via degradation using the innate machinery of the cell. This targeted protein degradation approach offers advantages over traditional drugs, including the potential to treat a wider range of diseases...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB