Sunday - April 6, 2025
Sunday - April 6, 2025
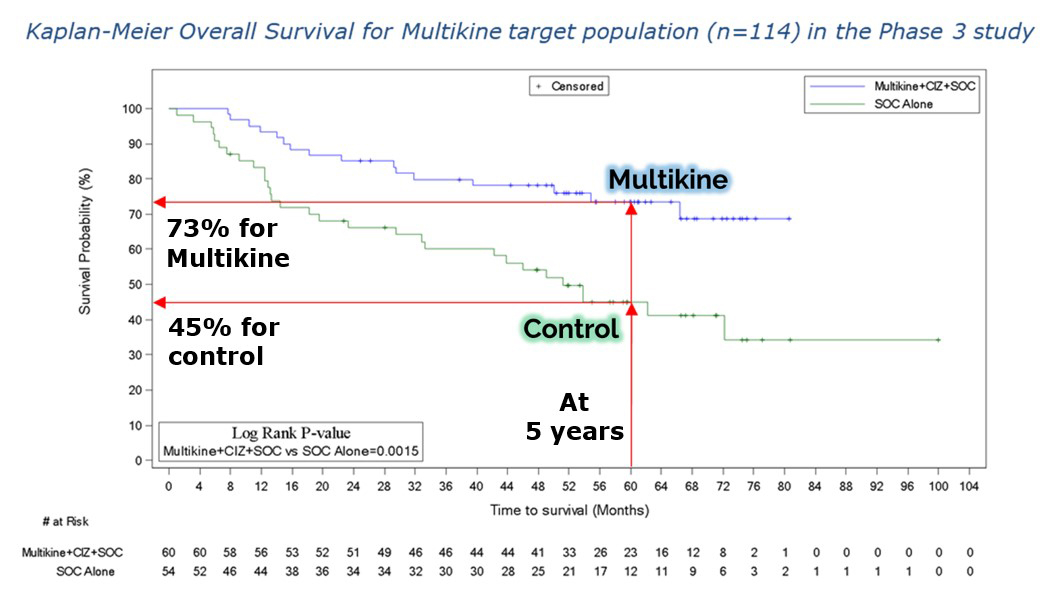
VIENNA, Va. / Oct 23, 2023 / Business Wire / CEL-SCI Corporation (NYSE American: CVM) today announced that it has finalized the selection criteria for the head and neck cancer target population to be treated with the Company’s immunotherapy drug Multikine (Leukocyte Interleukin, Injection). Five-year survival in the target population was 73% alive for Multikine-treated patients vs only 45% alive in the control who did not receive Multikine, with the five-year risk of death cut in half for Multikine-treated subjects in the target population versus the control. These data, which are statistically significant and accompanied by strong hazard ratios, are a crucial achievement on the path for the approval of Multikine. CEL-SCI presented the data for the first time at the European Society for Medical Oncology (ESMO) Congress in Spain on October 22, 2023. The selection criteria for this target population were developed based on the completed Phase 3 randomized controlled trial, advice from regulators, and advice from physician consultants associated with the University of California San Diego Cancer Center and Yale Medical School, recognized as among the nation’s most esteemed immuno-oncologists in head and neck cancer.
Patients within this Multikine target population can be readily identified upon diagnosis with tests that physicians routinely use in cancer screening—another crucial achievement towards making Multikine available for use, as it is given immediately after diagnosis and before any other treatment. Working with regulators, CEL-SCI can now write an appropriate approval label defining Multikine’s target population, which is supported by strong positive data from the Phase 3 trial. The target population is estimated to cover ~145,000 advanced primary head and neck cancer patients globally per year.
The final Multikine selection criteria are:
CEL-SCI’s ESMO poster reports three major new advancements supporting Multikine’s approvability:
A 28% 5-year absolute survival advantage (73% vs 45%, p=0.0015), hazard ratio 0.35 (95% CIs [0.18, 0.66], Wald p=0.0012). The p-value of 0.0015 makes these results strongly significant as a statistical matter. The hazard ratio for Multikine vs control was 0.35, meaning that the Multikine-treated patients had, on average, about 65% lower risk of death at any given time. CEL-SCI is not aware of any head and neck cancer therapy that has shown such a large 5-year survival benefit. In addition, the hazard ratio’s “95% confidence interval” upper bound was 0.66, which supports CEL-SCI’s confidence that a confirmatory trial of Multikine in the target population would likely be a success. These results and statistics support CEL-SCI’s confidence that the data should be sufficient for requesting immediate approval for Multikine to treat these patients who currently have an unmet need for better outcomes.
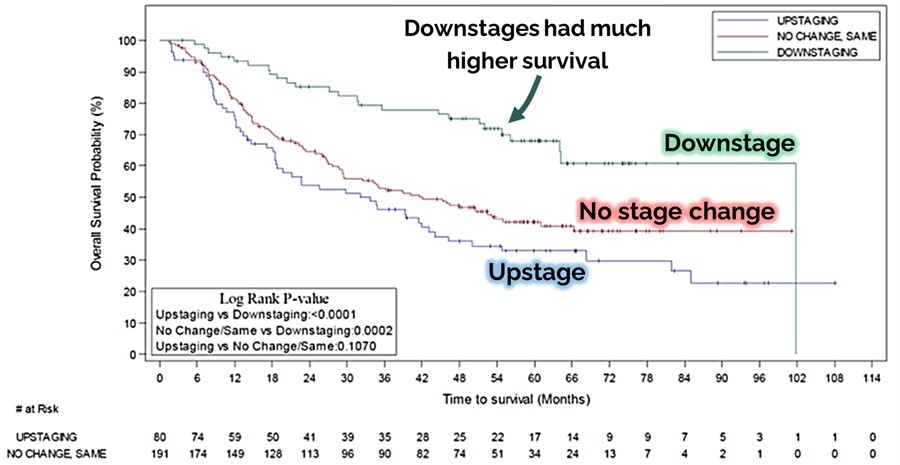
Another finding presented at ESMO is also very important because it predicts improved survival. This finding refers to pre-surgery disease downstaging, which is when the physician assesses a lower stage of disease after Multikine therapy compared to before Multikine therapy. Graph 2 above clearly indicates that Multikine-treated patients with downstaging (improved disease, the green top line) have much better survival. This data is strongly significant as a statistical matter, with a p-value below 0.0002.
Geert Kersten, Chief Executive Officer of CEL-SCI, said: “We are now able to identify upon diagnosis which patients are most likely to have pre-surgery responses to Multikine and improved survival, as seen in the new ESMO data. This is a new sign of hope for head and neck cancer patients and a critical milestone for Multikine towards achieving regulatory approval. Because Multikine is a neoadjuvant (pre-surgical) immunotherapy, identifying patients who benefit from Multikine upon diagnosis was very difficult, and we welcomed the assistance of regulators and expert physician consultants to help us. With this success announced at ESMO, we are eager to present these new data to regulators.”
John Cipriano, Senior VP of Regulatory Affairs at CEL-SCI, said: “Patients with this disease have a clear unmet medical need for better outcomes. Multikine’s demonstrated effects in the new intended target population are overwhelmingly large and strong as a statistical matter and support the observed Multikine efficacy. While Multikine has not yet been tested prospectively in the new target population, the Phase 3 data present a compelling case for immediate patient access to Multikine because the Phase 3 study results showed prospectively that Multikine led to pre-surgical responses which in turn led to longer life. Regulators understand that patients should not have to wait before gaining access to these benefits, particularly given Multikine’s safety profile and data that mechanistically and empirically supports the target population definition. There are specific pathways in Europe, the UK, Canada, and the U.S. for such approvals, and we are currently working towards this goal. We filed requests with the UK and European regulatory bodies within the past month, and we intend to press forward with these new results in Canada and the U.S. in the coming months. Therefore, we hope to receive approval in the target population without having to wait for completion of a confirmatory study.”
CEL-SCI’s Chief Scientific Officer, Eyal Talor, Ph.D., a co-author of CEL-SCI’s ESMO presentation, provided more detail on the data. “This year’s ESMO data presentation also relates to pre-surgery disease downstaging, which occurs, for example, when the disease stage goes down from Stage IV to Stage III during Multikine treatment before surgery. In the overall Phase 3 study population, we saw that patients with disease downstaging survived longer than those without. Therefore, we concluded that Multikine benefited patients who had downstaging before surgery. The target population selection criteria are designed to capture as many responder patients as possible, and indeed we saw a 35% rate of pre-surgical downstaging in the Phase 3 study subjects who met these selection criteria when treated with Multikine. This high rate of downstaging and tumor responses from Multikine led to the increase in overall survival seen in the target population and this is why the risk of death was cut in half for Multikine-treated patients as compared to control.”
Click here for a link to CEL-SCI’s ESMO 2023 poster presentation entitled: “Tumor Node stage shift following Leukocyte Interleukin, Injection (LI) neoadjuvant extends overall survival in treatment-naïve locally advanced primary squamous cell carcinoma of the oral cavity/soft palate.”
About CEL-SCI Corporation
CEL-SCI believes that boosting a patient’s immune system while it is still intact should provide the greatest possible impact on survival. Therefore, in the Phase 3 study, CEL-SCI studied patients who were newly diagnosed with locally advanced primary squamous cell carcinoma of the head and neck with the investigational product Multikine first, BEFORE they received surgery and radiotherapy or surgery plus concurrent radiotherapy and chemotherapy (the current standard of care for these patients). This approach is called neo-adjuvant. Most other cancer immunotherapies are administered only after conventional therapies have been tried and/or failed. Multikine (Leukocyte Interleukin, Injection) received Orphan Drug designation from the FDA for neoadjuvant therapy in patients with squamous cell carcinoma (cancer) of the head and neck.
Multikine is designed to help the immune system “target” the tumor at a time when the immune system is still relatively intact and thereby thought to be better able to mount an attack on the tumor. CEL-SCI has completed a 928 patient Phase 3 clinical trial in locally advanced primary head and neck cancer patients.
The Company has operations in Vienna, Virginia, and near/in Baltimore, Maryland.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. When used in this press release, the words "intends," "believes," "anticipated," "plans" and "expects," and similar expressions, are intended to identify forward-looking statements. Such statements are subject to risks and uncertainties that could cause actual results to differ materially from those projected. Such statements include, but are not limited to, statements about the terms, expected proceeds, use of proceeds and closing of the offering. Factors that could cause or contribute to such differences include an inability to duplicate the clinical results demonstrated in clinical studies, timely development of any potential products that can be shown to be safe and effective, receiving necessary regulatory approvals, difficulties in manufacturing any of the Company's potential products, inability to raise the necessary capital and the risk factors set forth from time to time in CEL-SCI's filings with the Securities and Exchange Commission, including but not limited to its report on Form 10-K for the year ended September 30, 2022. The Company undertakes no obligation to publicly release the result of any revision to these forward-looking statements which may be made to reflect the events or circumstances after the date hereof or to reflect the occurrence of unanticipated events.
* Multikine (Leukocyte Interleukin, Injection) is the trademark that CEL-SCI has registered for this investigational therapy. This proprietary name is subject to FDA review in connection with the Company's future anticipated regulatory submission for approval. Multikine has not been licensed or approved for sale, barter or exchange by the FDA or any other regulatory agency. Similarly, its safety or efficacy has not been established for any use.
| Last Trade: | US$0.22 |
| Daily Change: | 0.02 8.60 |
| Daily Volume: | 5,783,020 |
| Market Cap: | US$16.010M |
March 18, 2025 February 20, 2025 | |

Terns Pharmaceuticals is a clinical-stage biopharmaceutical company developing a portfolio of small-molecule product candidates to address serious diseases, including oncology and obesity. Terns’ pipeline contains three clinical stage development programs including GLP-1 receptor...
CLICK TO LEARN MORE
Amneal Pharmaceuticals is a fully-integrated essential medicines company. We make healthy possible through the development, manufacturing, and distribution of generic and specialty pharmaceuticals. The Company has a diverse portfolio of over 250 products in its Generics segment and is expanding across...
CLICK TO LEARN MOREEnd of content
No more pages to load