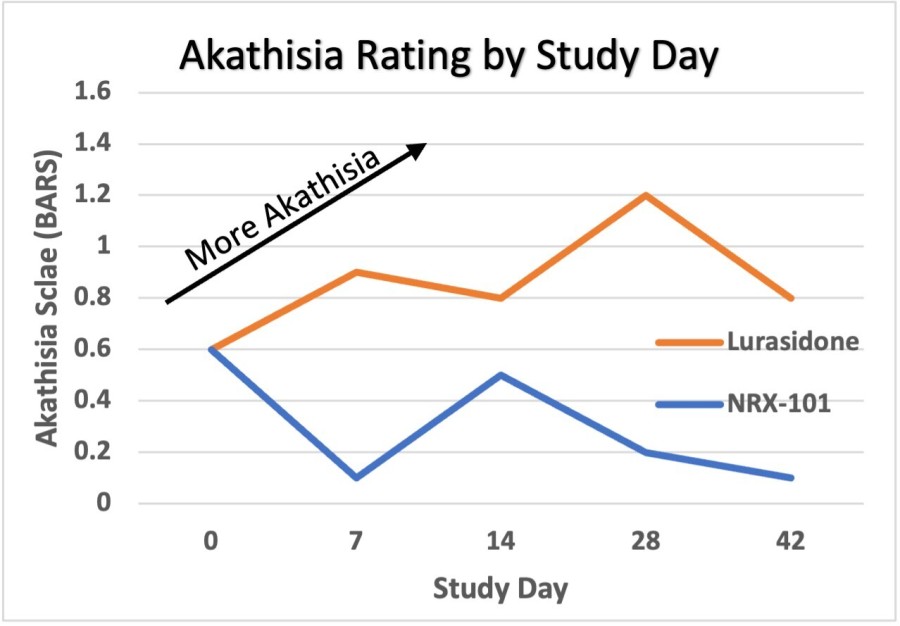
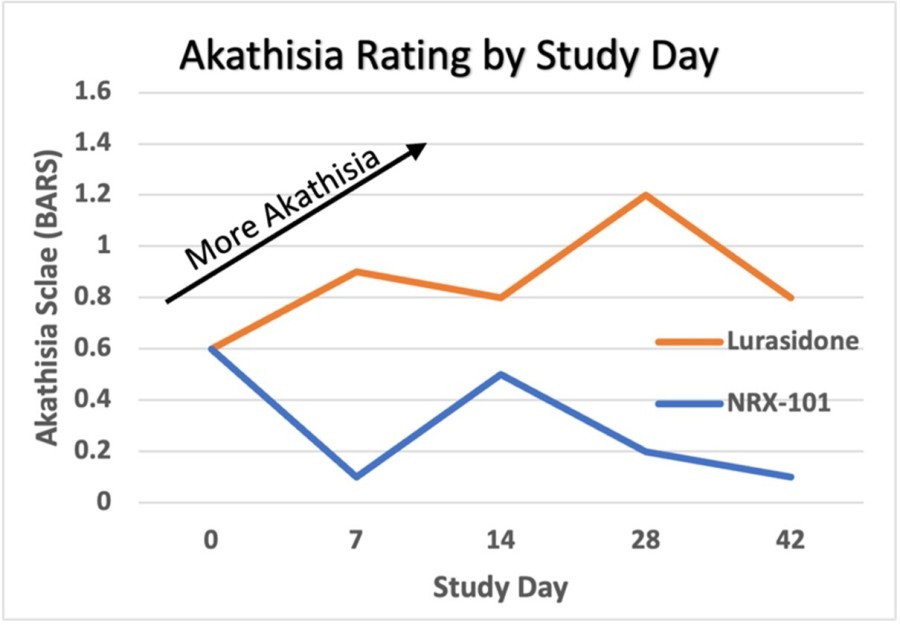
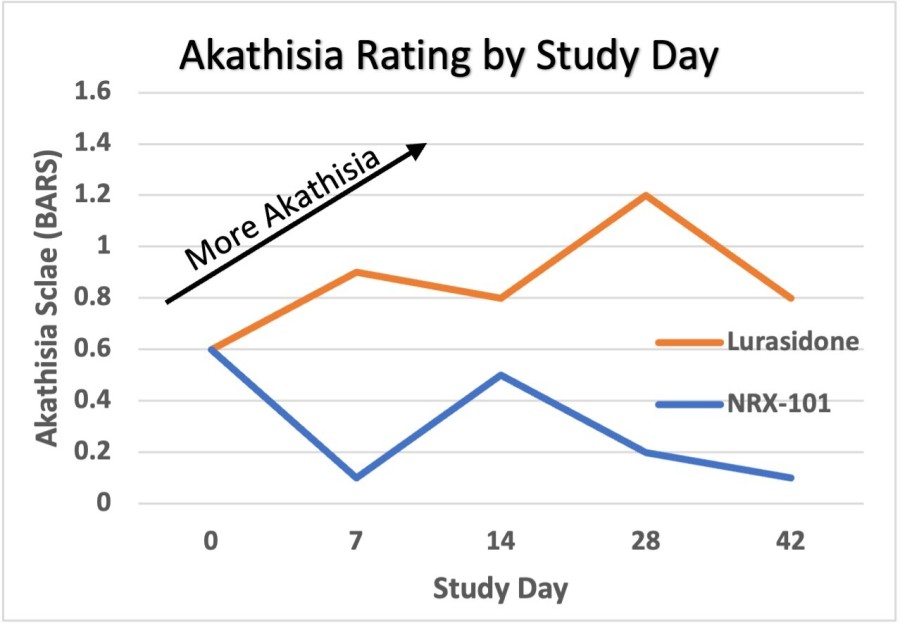
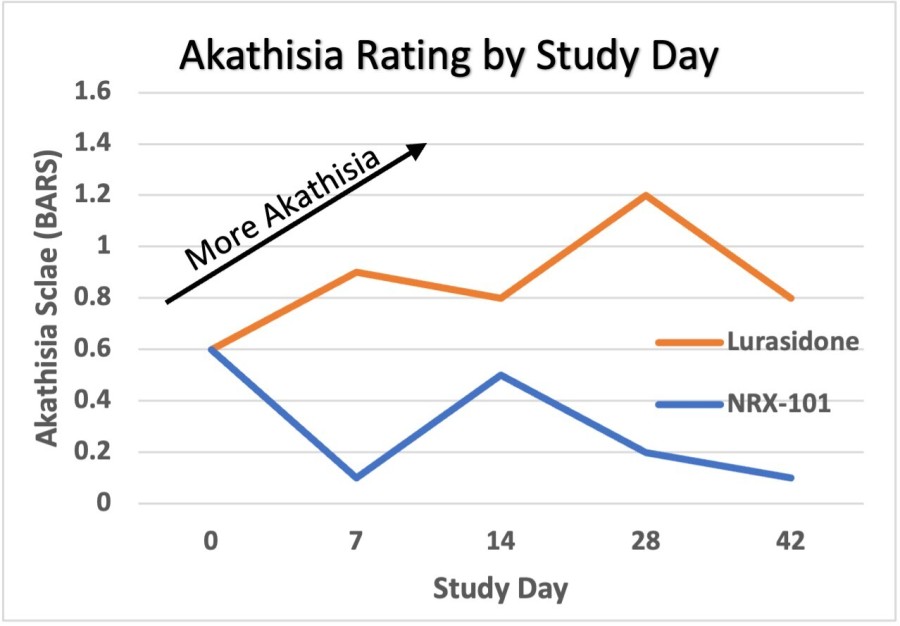
WILMINGTON, Del. , Nov. 26, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals", the "Company"), a clinical-stage biopharmaceutical company, today announced that Dr. Jonathan Javitt , Chairman, CEO and Chief Scientist will present at NobleCon20 - Noble Capital Markets' Twentieth Annual Emerging Growth Equity Conference at Florida Atlantic University , Executive Education Complex, in Boca... Read More