RADNOR, Pa., April 30, 2024 /PRNewswire/ -- NRx Pharmaceuticals, Inc. (Nasdaq: NRXP) ("NRx Pharmaceuticals", the "Company"), a clinical stage pharmaceutical company today that its Breakthrough Therapy designated investigational drug NRX-101 vs lurasidone demonstrated a promising, though not yet statistically significant 33% reduction in suicidality together with a 70% reduction (P=.076) reduction in symptoms of akathisia – a side effect of antidepressants that is closely linked to suicide and considered a medical emergency. Because of the high-risk nature of these patients, a placebo group could not be employed, and NRX-101, a fixed dose combination of D-cycloserine (DCS) and lurasidone, was compared to lurasidone alone (the standard of care). In the Company's previously published STABIL-B trial (STABIL-B), NRX-101 was demonstrated to be superior to lurasidone in reducing both depression and suicidality after ketamine while showing a trend towards reducing akathisia (a side effect involving restlessness and agitation that is considered a warning sign of impending suicide). In this trial, without prior use of ketamine, NRX-101 and lurasidone were comparable in their effect on depression. The trial was a randomized, prospective, double-blind study conducted at multiple sites in the Unites States whose protocol and statistical analysis plan may be viewed on www.clinicaltrials.gov (NCT03395392).
"We are gratified by these results, which extend the findings of the STABIL-B trial in suggesting that NRX-101 has the potential to be the first oral antidepressant to decrease potential for suicide, whereas all currently approved oral antidepressants are known to increase the risk of suicide," said Prof. Jonathan Javitt, MD, MPH, the Company's Chairman and Chief Scientist. "Should these findings be confirmed in a registrational trial of 300 patients, NRX-101 has the potential to represent a paradigm-changing blockbuster drug. The finding of a dramatic difference in akathisia was also seen in the STABIL-B trial and provides important mechanistic support for the difference seen on the Columbia Suicide Severity Rating Scale. Many of the patients who tragically die from suicide in bipolar depression are taking traditional antidepressants at the time of their death, a tragedy we have seen within the families of our investors and board members, as well as the many patients we have known. If today's findings are replicated in a registration-sized trial, we will change the world for patients who currently have a 50% lifetime risk of suicide attempt, a 20% lifetime risk of death by suicide, and whose only approved treatment option today is electroshock therapy."
"These findings are consistent with our original Phase 2 objectives and promising zone methodology in terms of a demonstrable advantage of NRX-101 compared to the standard of care in treating patients with bipolar depression who are known to be at high risk of suicide. We originally proposed to test suicidality, rather than depression as the primary endpoint for this trial and took the advice of senior FDA leadership that demonstrating a difference in suicidality might be too challenging. Today's findings demonstrate that differences in suicidality and akathisia can be demonstrated compared to best available antidepressant therapy in a properly sized registration trial and that superiority over placebo on the depression scale may readily be demonstrated in a less acute patient population where it would be safe to do so," said Dr. Philip Lavin, the study's Lead Methodologist. Dr. Lavin is one of the world's most widely published statisticians who has led the approval/clearance of more than 80 drugs, devices, and biologics.
In the current study, without prior use of ketamine, NRX-101 and lurasidone exhibited comparable antidepressant effects, each reducing depression (the primary endpoint) on the Montgomery Asberg Depression Rating Scale (MADRS) by about 50% from baseline. Lurasidone is known to reduce symptoms of depression by approximately 4 points in multiple registration trials compared to placebo.
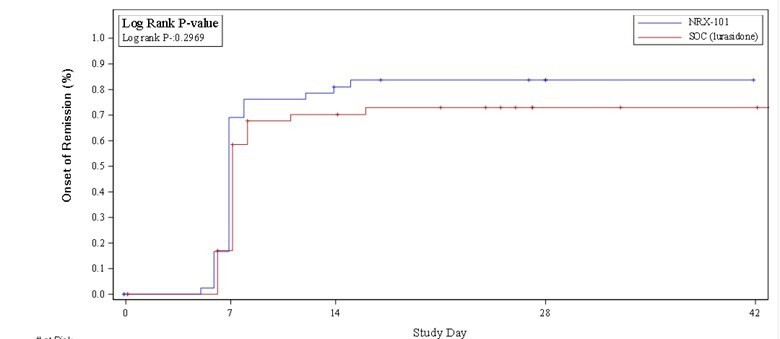
Analysis of suicidality using the Columbia Suicide Severity Rating Scale (C-SSRS) demonstrated a sustained 33% advantage in remission from suicidality favoring NRX-101 (see figure). This difference was not statistically significant at the phase 2 sample size but met the study's original promising zone criteria and, if sustained in a registration trial of 300 or more patients, would be powered to yield a statistically significant result. The reduction in suicidality is comparable to that demonstrated after ketamine, both in the Company's STABIL-B trial and in an independently conducted trial comparing DCS to placebo after ketamine (Chen, et. al.). A meaningful remission in suicidality has not been demonstrated with any prior oral antidepressant drug – indeed, antidepressant drugs carry a Black Box warning of increased suicide risk.
Reduction in akathisia was first identified in the laboratory as a distinguishing feature of DCS and is the basis of the approved claims in the Company's Composition of Matter patents. Akathisia is often characterized as a state of agitation and motor restlessness that is associated with particularly impulsive and tragically effective attempts at suicide, such as hanging, shooting, jumping from buildings and in front of vehicles and trains. In this trial, a 75% relative difference was seen on the Barnes Akathisia Rating Scale (BARS), with two-sided P=0.076, which would be expected to achieve significance in a properly powered registration-sized trial. While reduction in akathisia is not proposed as a primary labeled indication, continued finding of a statistically significant reduction in this side effect would be highly supportive of a demonstrated primary endpoint of reduced suicidality and would provide clinical corroboration.
Based on these findings and widespread adoption of ketamine as initial treatment for suicidal depression, the Company believes that NRX-101 may become the drug of choice for potentiating the effect of ketamine in patients with acute and subacute suicidality. The FDA recently affirmed to the Company that the Special Protocol Agreement for this indication remains in place, subject to the Company filing a New Drug Approval for ketamine, which is expected by July 2024. Moreover the Company aims to explore the role of NRX-101 as primary treatment for the much larger population (approximately 7 million in the US) of patients with bipolar depression who do not have active suicidality and, therefore, do not require prior treatment with intravenous ketamine.
About NRx Pharmaceuticals
NRx Pharmaceuticals is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. The Company is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain. NRx has partnered with Alvogen and Lotus around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRx has recently announced plans to submit a New Drug Application for HTX-100 (IV ketamine), through Hope Therapeutics, in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRx was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
About HOPE Therapeutics, Inc.
HOPE Therapeutics, Inc. (www.hopetherapeutics.com) is a Specialty Pharmaceutical Company, wholly-owned by NRX Pharmaceuticals focused on development and marketing of an FDA-approved form of intravenous ketamine for the treatment of acute suicidality and depression together with a digital therapeutic-enabled platform designed to augment and preserve the clinical benefit of NMDA-targeted drug therapy.
Notice Regarding Forward-Looking Statements
The information contained herein includes forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. These statements include, among others, statements regarding the proposed public offering and the timing and the use of the proceeds from the offering. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as "may," "will," "should," "would," "expect," "plan," "believe," "intend," "look forward," and other similar expressions among others. These statements relate to future events or to the Company's future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause the Company's actual results to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. You should not place undue reliance on forward-looking statements since they involve known and unknown risks, uncertainties and other factors which are, in some cases, beyond the Company's control and which could, and likely will, materially affect actual results, levels of activity, performance or achievements. Any forward-looking statement reflects the Company's current views with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to the Company's operations, results of operations, growth strategy and liquidity. More detailed information about the Company and the risk factors that may affect the realization of forward-looking statements is set forth in the Company's most recent Annual Report on Form 10-K and other filings with the Securities and Exchange Commission. Investors and security holders are urged to read these documents free of charge on the SEC's website at http://www.sec.gov. Except as may be required by applicable law, The Company assumes no obligation to publicly update or revise these forward-looking statements for any reason, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, whether as a result of new information, future events or otherwise.

| Last Trade: | US$1.51 |
| Daily Change: | -0.005 -0.33 |
| Daily Volume: | 675,211 |
| Market Cap: | US$18.200M |
December 30, 2024 November 25, 2024 November 14, 2024 October 30, 2024 October 03, 2024 | |

Recursion Pharmaceuticals is a clinical stage TechBio company leading the space by decoding biology to industrialize drug discovery. Enabling its mission is the Recursion OS, a platform built across diverse technologies that continuously expands one of the world’s largest....
CLICK TO LEARN MORE
Amneal Pharmaceuticals is a fully-integrated essential medicines company. We make healthy possible through the development, manufacturing, and distribution of generic and specialty pharmaceuticals. The Company has a diverse portfolio of over 250 products in its Generics segment and is expanding across...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB