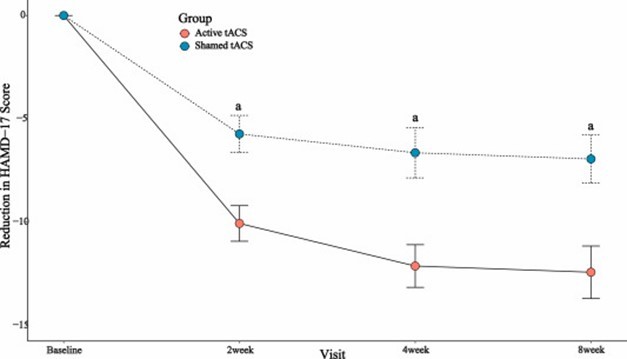
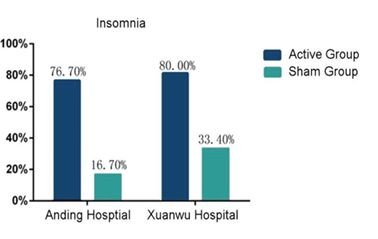
HOUSTON, TX, Nov. 11, 2024 (GLOBE NEWSWIRE) -- Nexalin Technology, Inc. (Nasdaq: NXL; NXLIW) (the “Company” or “Nexalin”) today released a letter to shareholders from its CEO Mark White: Dear Shareholders, I am pleased to provide an update on Nexalin Technologies’ recent milestones, which underscore our commitment to advancing our patented Deep Intracranial Frequency Stimulation (DIFS) technology for the treatment of... Read More