HOUSTON, TEXAS, Dec. 18, 2023 (GLOBE NEWSWIRE) -- Nexalin Technology, Inc. (the “Company” or “Nexalin”) (Nasdaq: NXL; NXLIW) today reported the results of a study in patients with treatment-resistant depression (TRD), concerning the potential therapeutic benefits of its second-generation (Gen-2), 15 milliamp (mA) neurostimulation device, which indicated a substantial and statistically significant benefit in patients.
The clinical trial was funded by the Company’s joint venture partner, Wider Come Limited (“Wider”), and its related companies, and was conducted at the Xuanwu Hospital, Capital Medical University in Beijing, China. The results were also published in General Psychiatry, an open-source, peer-reviewed scientific journal. The published results of the study concluded that repeated treatment with Nexalin’s neurostimulation device suggests an acute effect in reducing depressive symptoms in patients with TRD. In addition, no adverse events were observed during treatment.
Major depressive disorder (MDD), also known as clinical depression, is a mental health condition that affects mood, behavior, appetite, and sleep. According to a 2022 National Survey on Drug Use and Health, an estimated 22.47 million adults, or 8.8% of all US adults in the United States, had at least one major depressive episode. According to Future Market Insights, the MDD treatment market is forecast to reach US$ 14.96 billion by 2032 from US$ 11.51 billion in 2022. It is estimated that one-third of patients with MDD will not respond to two or more antidepressant drugs with different mechanisms with these patients being referred to as having treatment-resistant depression (TRD). American military veterans are particularly at risk for TRD, with nearly one in seven suffering from MDD considered to have TRD. In part for this reason, the President’s 2023 Budget Request included $13.9 billion towards Veterans Mental Healthcare services, an increase of 14% above the 2022 Budget.
As part of the clinical study, 7 migraine patients were treated at the Xuanwu Hospital. Treatment was administered for 4 consecutive weeks via the forehead and both mastoid (twice per day, 5 days a week). Efficacy and adverse reactions were assessed at a 2-week screening/baseline period followed by a 4-week treatment phase. The study concluded that twice daily 15mA tACS, a unique form of non-invasive brain stimulation, offers an acute effective intervention for patients with TRD. The study showed that all patients with TRD had a significant reduction in depression symptoms after the 4-week treatment, and all of them achieved a clinical response (defined as HAMD-17/ MADRS scores that decreased by 50% or more from the baseline).
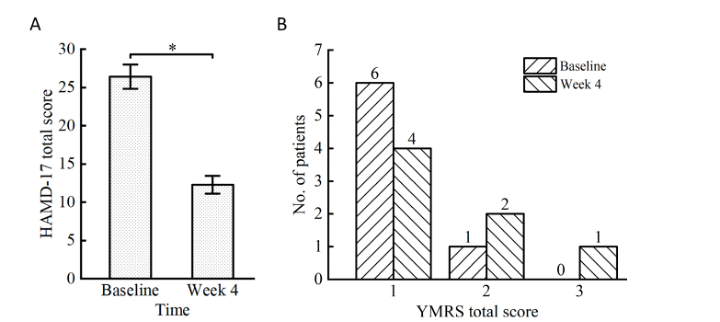 Figure 1. Clinical outcomes. (A) The mean HAMD-17 total scores significantly decreased from baseline to week 4. The error bars represent the SD. The asterisk (*) indicates a statistically significant difference with p<0.05. (B) Total YMRS scores at baseline and week 4. HAMD-17, 17-item Hamilton Rating Scale for Depression; No. number; SD, standard deviation; YMRS, Young Mania Rating Scale.
Figure 1. Clinical outcomes. (A) The mean HAMD-17 total scores significantly decreased from baseline to week 4. The error bars represent the SD. The asterisk (*) indicates a statistically significant difference with p<0.05. (B) Total YMRS scores at baseline and week 4. HAMD-17, 17-item Hamilton Rating Scale for Depression; No. number; SD, standard deviation; YMRS, Young Mania Rating Scale.
The previous 8-week randomized, double-blind, sham-controlled trial evaluating 100 patients with major depressive disorder (MDD), published in the international journal, Brain, indicated that patients receiving treatment with the Company’s Gen-2, 15mA neurostimulation device had better remission and response rates than the sham group. Compared with sham treatment, almost all depressive symptoms among the active treatment showed significant improvement. Moreover, there was no significant statistical difference in adverse events between the two groups.
Mark White, CEO of Nexalin Technology, stated, “We are pleased to report the results of this study in patients with treatment-resistant depression (TRD), which have been featured in a leading peer-reviewed journal. These results reinforce the growing body of clinical evidence supporting the potential of Nexalin's new advanced waveform to help combat the ongoing global mental health epidemic. We believe the data provides further evidence of the significant impact of our non-invasive, drug-free device on improving mental healthcare outcomes among patients affected with TRD. No significant adverse effects were reported, which is especially noteworthy given the growing number of patients seeking non-pharmacological treatment options. Overall, we remain committed to our mission of bringing our new, effective, and drug-free therapy to patients with mental health issues in the United States and around the world.”
About Nexalin Technology, Inc.
Nexalin designs and develops innovative neurostimulation products to uniquely and effectively help combat the ongoing global mental health epidemic. All of Nexalin’s products are non-invasive and undetectable to the human body and developed to provide relief to those afflicted with mental health issues. Nexalin utilizes bioelectronic medical technology to treat mental health issues. Nexalin believes its neurostimulation medical devices can penetrate structures deep in the mid-brain that are associated with mental health disorders. Nexalin believes the deeper penetrating waveform in its next-generation devices will generate enhanced patient response without any adverse side effects. The Nexalin Gen-2 15 milliamp (mA) neurostimulation device was recently approved in China by the National Medical Products Administration (NMPA) for the treatment of insomnia and depression. Additional information about the Company is available at: https://nexalin.com/.
FORWARD-LOOKING STATEMENTS
This press release contains statements that constitute "forward-looking statements," These statements relate to future events or Nexalin’s future financial performance. Any statements that refer to expectations, projections or other characterizations of future events or circumstances or that are not statements of historical fact (including without limitation statements to the effect that Nexalin or its management “believes”, “expects”, “anticipates”, “plans”, “intends” and similar expressions) should be considered forward looking statements that involve risks and uncertainties which could cause actual events or Nexalin’s actual results to differ materially from those indicated by the forward-looking statements. Forward-looking statements are subject to numerous conditions, many of which are beyond the control of the Company, including those set forth in the Risk Factors section of the Company's Report on Form 10-K for the year ended December 31, 2022 and other filings as filed with the Securities and Exchange Commission. Copies of such filings are available on the SEC's website, www.sec.gov. Such forward-looking statements are made as of the date hereof and may become outdated over time. Such forward-looking statements are made as of the date hereof and may become outdated over time. The Company undertakes no obligation to update these statements for revisions or changes after the date of this release, except as required by law.
Contact:
Crescendo Communications, LLC
Tel: (212) 671-1020
Email: This email address is being protected from spambots. You need JavaScript enabled to view it.
Nexalin Clinical Outcomes Data Table

| Last Trade: | US$2.63 |
| Daily Change: | -0.43 -14.05 |
| Daily Volume: | 888,157 |
| Market Cap: | US$33.820M |
November 01, 2024 October 22, 2024 | |

Compass Therapeutics is a clinical-stage, oncology-focused biopharmaceutical company developing proprietary antibody-based therapeutics to treat multiple human diseases. The company's scientific focus is on the relationship between angiogenesis, the immune system, and tumor growth...
CLICK TO LEARN MORE
Terns Pharmaceuticals is a clinical-stage biopharmaceutical company developing a portfolio of small-molecule product candidates to address serious diseases, including oncology and obesity. Terns’ pipeline contains three clinical stage development programs including GLP-1 receptor...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB