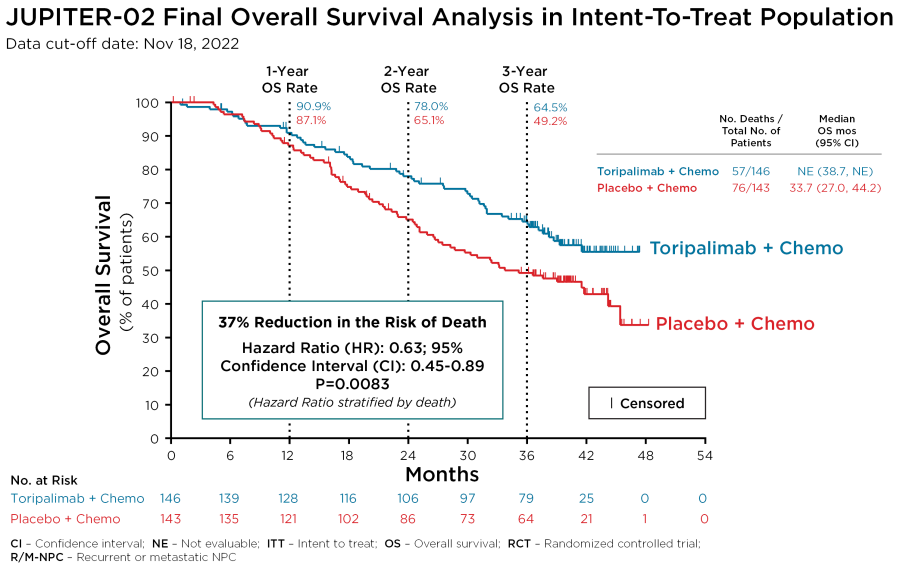
Randomized Phase 2 study initiated evaluating the combination of casdozokitug, toripalimab and bevacizumab in patients with liver cancer REDWOOD CITY, Calif., Dec. 18, 2024 (GLOBE NEWSWIRE) -- Coherus BioSciences, Inc. (“Coherus,” NASDAQ: CHRS) today announced that an abstract highlighting final clinical and biomarker data from its Phase 2 clinical trial evaluating casdozokitug (casdozo), a selective and potent... Read More