REDWOOD CITY, Calif., July 13, 2023 (GLOBE NEWSWIRE) -- Coherus BioSciences, Inc. (“Coherus”, Nasdaq: CHRS) and specialty pharmacy Superior Biologics, Inc. (“Superior Biologics”) today announced a national distribution agreement for YUSIMRY™ (adalimumab-aqvh) at a price of $995 per carton of two autoinjectors, a discount of more than 85% compared to Humira® (adalimumab), currently priced at $6,922 per carton of two pens.
“Coherus is pleased to work with Superior Biologics for nationwide distribution of YUSIMRY, providing patients and their plan sponsors access to a high-quality, safe, and effective treatment at significant savings compared to Humira,” said Denny Lanfear, Coherus’ Chairman and Chief Executive Officer. “Superior Biologics is an ideal partner for the distribution of YUSIMRY based on their commitment to quality patient service and care, their extensive experience working with manufacturers, patients, physicians, and plan sponsors, and their nationwide reach. Through this unique distribution partnership, we can bring YUSIMRY immediately to patients throughout the United States.”
“Patients deserve more affordable access to specialty drugs to treat their life-altering illnesses,” said Robert Cappa, RPh, President of Superior Biologics. “The national distribution of YUSIMRY through this agreement fulfills our shared vision with Coherus of delivering affordable solutions to patients with health challenges. This distribution partnership is an important and innovative step toward creating new options in the marketplace for patients to access specialty drugs, starting with the delivery of YUSIMRY at a fraction of the cost of Humira. It’s a game-changer for both patients and employers who have faced significant challenges from the skyrocketing costs of specialty drugs.”
Superior Biologics specializes in high-touch patient services and delivers quality care for a variety of disease states including rheumatology, gastroenterology, and neurology. An unmatched track record for meeting patients’ needs has led to high levels of customer satisfaction and allowed Superior Biologics to become a trusted resource for prescribing physicians.
Coherus first announced the release of YUSIMRY on July 3, 2023 at an annual price of less than $13,000 compared to nearly $90,000 for Humira® (adalimumab) based on the company’s investment in robust large-scale manufacturing, passing the savings on directly to patients in need. The agreement is the first between the two companies, marking an innovative distribution model that will serve patients effectively and allow employers to achieve significant savings in their specialty drug costs.
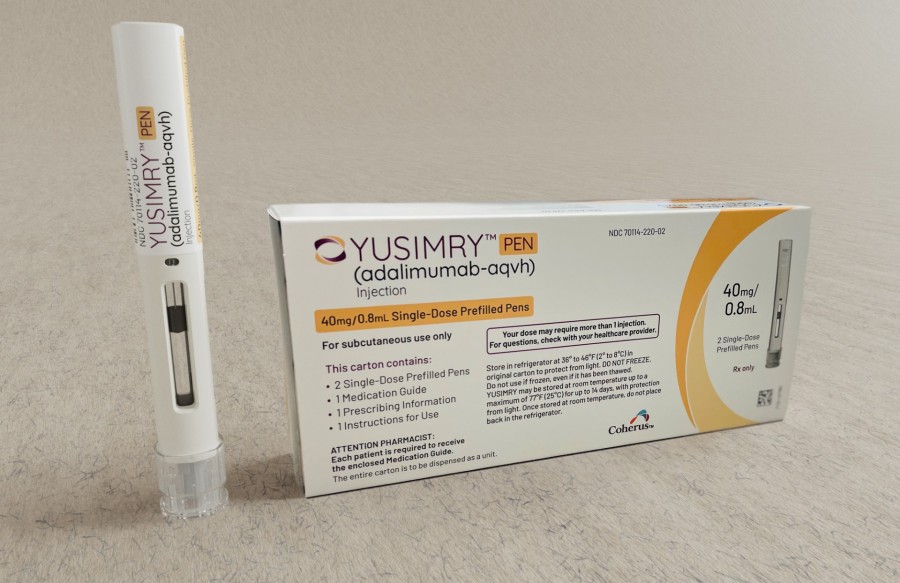
About YUSIMRY
YUSIMRY™ (adalimumab-aqvh), a biosimilar of Humira® (adalimumab), is a tumor necrosis factor (“TNF”) blocker indicated to reduce the signs and symptoms of rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, and ankylosing spondylitis, and to treat Crohn’s disease, ulcerative colitis, plaque psoriasis, and hidradenitis suppurativa.
IMPORTANT SAFETY INFORMATION AND INDICATIONS
YUSIMRY™ (adalimumab-aqvh) is biosimilar* to Humira® (adalimumab injection).
WARNING: SERIOUS INFECTIONS AND MALIGNANCY
See full prescribing information for complete boxed warning.
Patients treated with YUSIMRY are at increased risk for developing serious infections that may lead to hospitalization or death. Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids.
Discontinue YUSIMRY if a patient develops a serious infection or sepsis.
Reported infections include:
Carefully consider the risks and benefits of treatment with YUSIMRY prior to initiating therapy in patients: 1. with chronic or recurrent infection; 2. who have been exposed to TB; 3. with a history of opportunistic infection; 4. who resided in or traveled in regions where mycoses are endemic; 5. with underlying conditions that may predispose them to infection. Monitor patients closely for the development of signs and symptoms of infection during and after treatment with YUSIMRY, including the possible development of TB in patients who tested negative for latent TB infection prior to initiating therapy.
Do not start YUSIMRY during an active infection, including localized infections.
MALIGNANCY
Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with TNF blockers, including adalimumab. Postmarketing cases of hepatosplenic T-cell lymphoma (HSTCL), a rare type of T-cell lymphoma, have been reported in patients treated with TNF blockers, including adalimumab. These cases have had a very aggressive disease course and have been fatal. The majority of reported TNF blocker cases have occurred in patients with Crohn’s disease or ulcerative colitis and the majority were in adolescent and young adult males. Almost all of these patients had received treatment with azathioprine or 6-mercaptopurine concomitantly with a TNF blocker at or prior to diagnosis. It is uncertain whether the occurrence of HSTCL is related to use of a TNF blocker or a TNF blocker in combination with these other immunosuppressants.
HYPERSENSITIVITY
HEPATITIS B VIRUS REACTIVATION
NEUROLOGIC REACTIONS
HEMATOLOGIC REACTIONS
CONGESTIVE HEART FAILURE
AUTOIMMUNITY
IMMUNIZATIONS
ADVERSE REACTIONS
INDICATIONS
*Biosimilar means that the biological product is approved based on data demonstrating that it is highly similar to an FDA-approved biological product, known as a reference product, and that there are no clinically meaningful differences between the biosimilar product and the reference product. Biosimilarity of YUSIMRY has been demonstrated for the condition(s) of use (e.g., indication(s), dosing regimen(s)), strength(s), dosage form(s), and route(s) of administration described in its Full Prescribing Information.
Click for YUSIMRY™ Full Prescribing Information, including Boxed Warning and Medication guide
About Coherus BioSciences
Coherus is a commercial-stage biopharmaceutical company focused on the research, development and commercialization of innovative immunotherapies to treat cancer. Coherus’ strategy is to build a leading immuno-oncology franchise funded with cash generated through net sales of its diversified portfolio of FDA-approved therapeutics.
In 2021, Coherus in-licensed toripalimab, an anti-PD-1 antibody, in the United States and Canada. The Biologics License Application for toripalimab in combination with chemotherapy as treatment for recurrent or metastatic nasopharyngeal carcinoma is currently under review by the FDA.
Coherus markets UDENYCA® (pegfilgrastim-cbqv), a biosimilar of Neulasta®, CIMERLI® (ranibizumab-eqrn), a biosimilar of Lucentis®, and YUSIMRY™ (adalimumab-aqvh), a biosimilar of Humira®.
About Superior Biologics
Founded in 2012, Superior Biologics, Inc. is a leading national provider of specialty pharmacy and home infusion therapy services to patients suffering from a wide variety of chronic diseases. Superior Biologics provides innovative and cost-effective pharmacy and in-home nursing infusion services that result in optimal therapeutic outcomes and deliver best-in-class patient results. By offering the highest levels of service and quality, Superior Biologics has become a trusted partner of top physicians and thought leaders from the most prestigious medical centers and universities across the country. Superior Biologics’ pharmacies are accredited by the Utilization Review Accreditation Commission (URAC), The Joint Commission (TJC) and the Accreditation Commission for HealthCare (ACHC).
Forward-Looking Statements
Except for the historical information contained herein, the matters set forth in this press release are forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995, including, but not limited to, statements regarding Coherus’ ability to build its immuno-oncology franchise to achieve a leading market position; Coherus’ ability to generate cash; Coherus’ investment plans; Coherus’ expectations for the effectiveness of its distribution model.
Such forward-looking statements involve substantial risks and uncertainties that could cause Coherus’ actual results, performance, or achievements to differ significantly from any future results, performance or achievements expressed or implied by the forward-looking statements. Such risks and uncertainties include, among others, risks related to our existing and potential collaboration partners, the risks and uncertainties inherent in the clinical drug development process; risks of the drug development position of Coherus’ competitors; the risks and uncertainties of the regulatory approval process, including the speed of regulatory review, the risk of FDA review issues; and the risks and uncertainties of possible litigation. All forward-looking statements contained in this press release speak only as of the date of this press release. Coherus undertakes no obligation to update or revise any forward-looking statements. For a further description of the significant risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to Coherus’ business in general, see Coherus’ Quarterly Report on Form 10-Q for the fiscal quarter ended March 31, 2023, filed with the Securities and Exchange Commission on May 8, 2023, including the section therein captioned “Risk Factors” and in other documents that Coherus files with the Securities and Exchange Commission.
UDENYCA®, CIMERLI® and YUSIMRY™, whether or not appearing in large print or with the trademark symbol, are trademarks of Coherus, its affiliates, related companies or its licensors or joint venture partners unless otherwise noted. Trademarks and trade names of other companies appearing in this press release are, to the knowledge of Coherus, the property of their respective owners.
Coherus Contact Information:
For Investors:
Marek Ciszewski, J.D., SVP Investor Relations
This email address is being protected from spambots. You need JavaScript enabled to view it.
For Media:
John Brandt, Rokk Solutions
This email address is being protected from spambots. You need JavaScript enabled to view it.
202-805-1830
Superior Biologics Contact information:
Peter Fricke
This email address is being protected from spambots. You need JavaScript enabled to view it.
(847) 924-2584

| Last Trade: | US$1.11 |
| Daily Change: | 0.29 35.12 |
| Daily Volume: | 8,709,519 |
| Market Cap: | US$127.880M |
August 08, 2024 June 27, 2024 May 30, 2024 | |

Chimerix is on a mission to develop medicines that meaningfully improve and extend the lives of patients facing deadly diseases. The company is devoted to filling gaps in the treatment paradigm. Chimerix’s most advanced clinical-stage program is in development for H3 K27M-mutant glioma....
CLICK TO LEARN MORE
Immix Biopharma is a clinical-stage biopharmaceutical company pioneering a novel class of CAR-T cell therapies and Tissue-Specific Therapeutics targeting oncology and immuno-dysregulated diseases with >75 patients treated to-date. Our lead cell therapy asset is NXC-201...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB