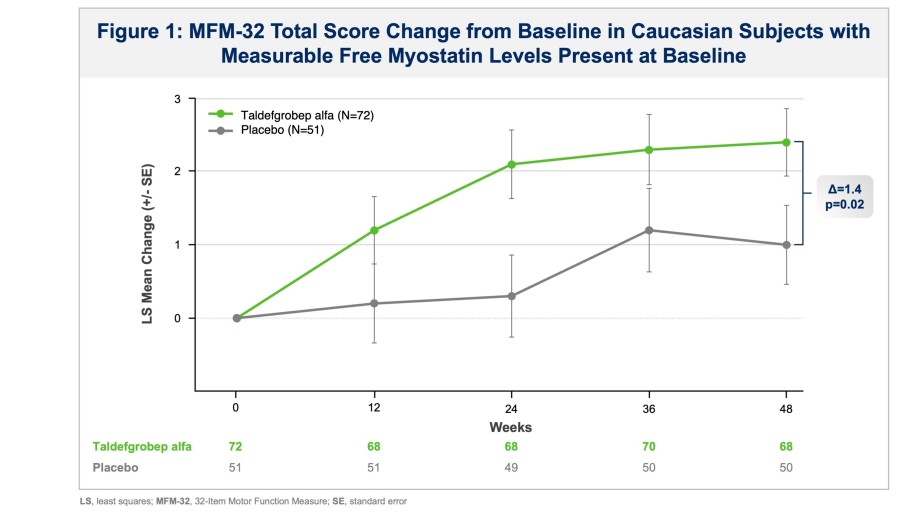
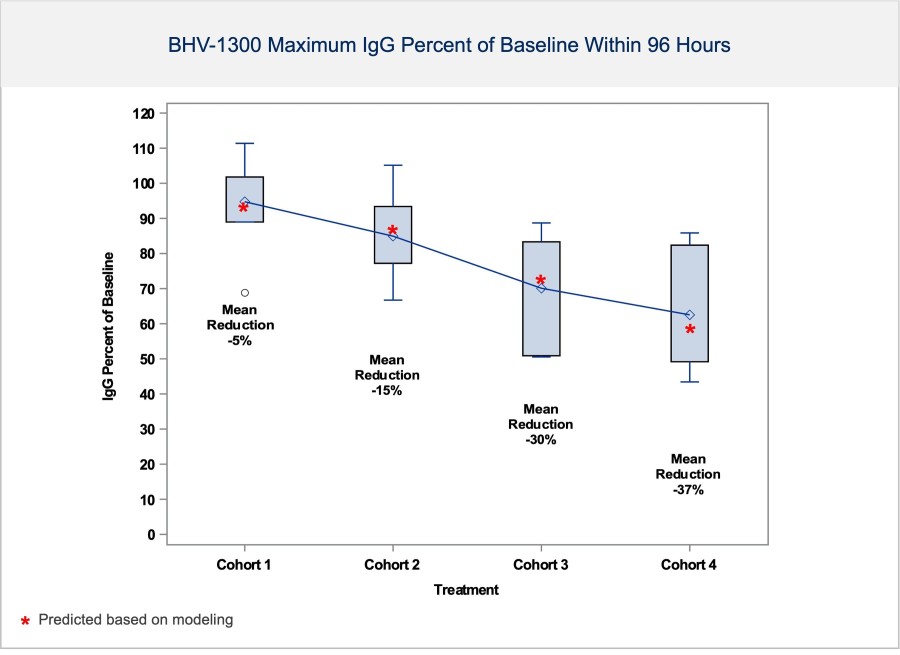
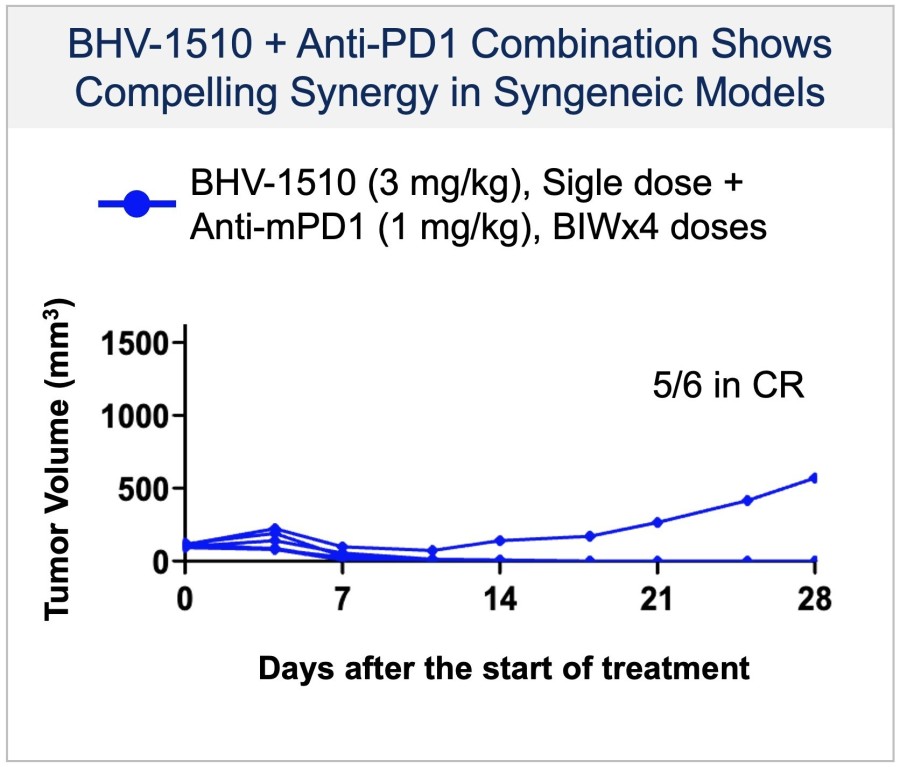
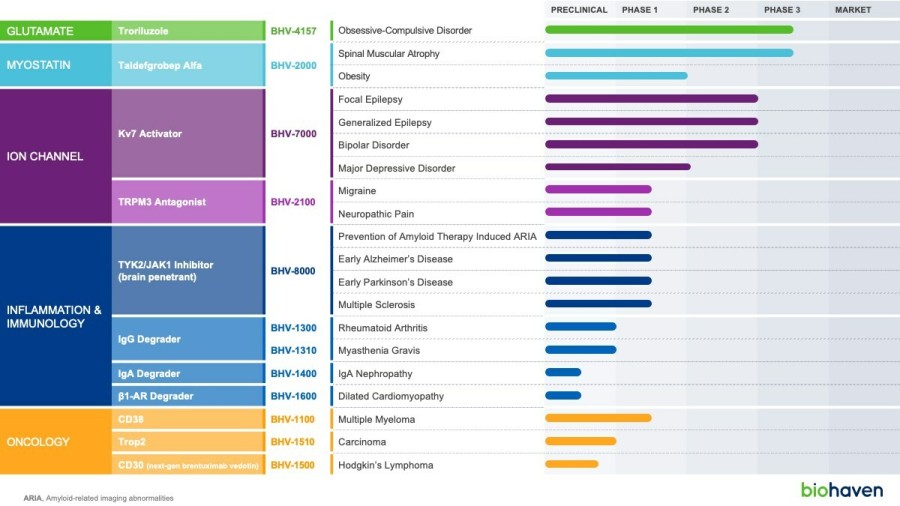
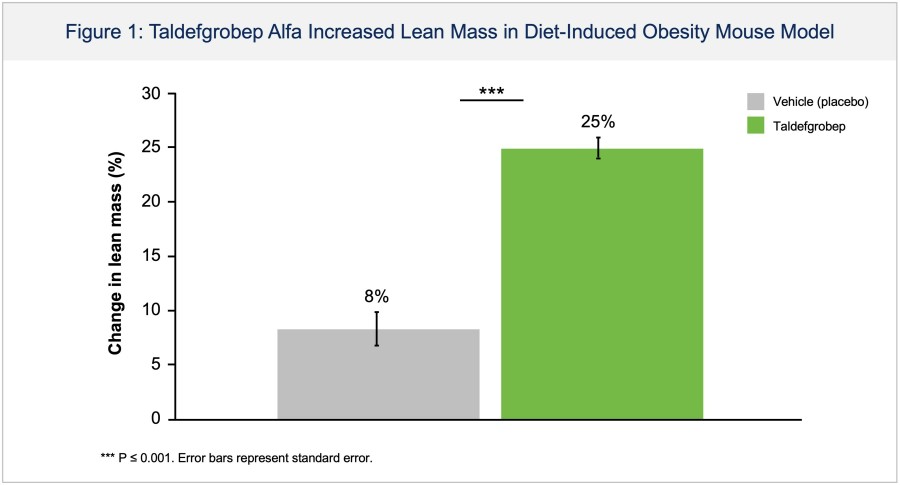
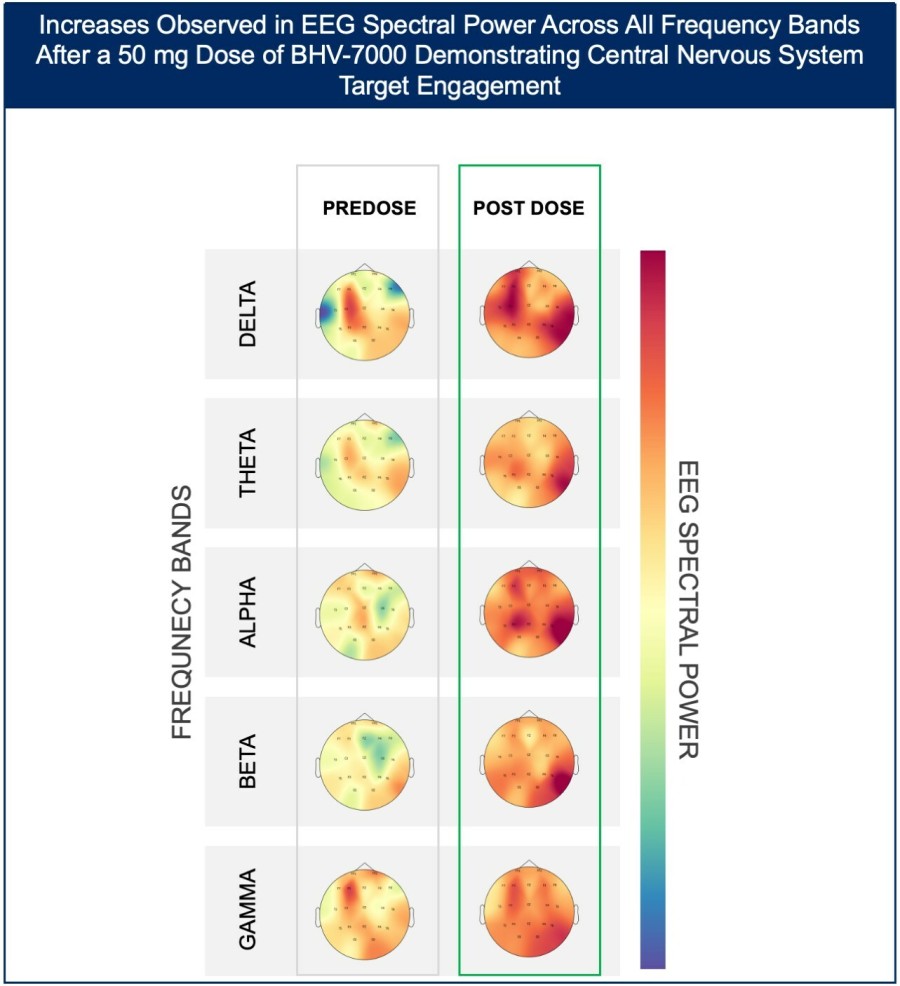
BHV-1300 achieved deep lowering of targeted IgG, with reductions > 60% in the lowest subcutaneous dose tested in the MAD. Subcutaneous BHV-1300 achieved rapid and progressive lowering of IgG within hours of each weekly dose administration, and pharmacodynamic effects were sustained relative to baseline over the four-week... Read More