NEW HAVEN, Conn., May 29, 2024 /PRNewswire/ -- Biohaven Ltd. (NYSE: BHVN) (Biohaven or the Company), a global clinical-stage biopharmaceutical company focused on the discovery, development and commercialization of life-changing therapies, provides an overview of its development and regulatory advances across multiple therapeutic areas, and highlights the progress of its innovative degrader pipeline at the Company's 2024 Investor R&D Day today, held concurrently with the Yale Innovation Summit in New Haven, Connecticut. Members of Biohaven's senior management team and key opinion leaders will share updates with investors and research analysts. The presentation slides will be available on the Events and Presentations page of the Biohaven website. An audio webcast will be available within 24 hours of the presentation.
The clinical progress, regulatory updates and pipeline developments at Biohaven's R&D Day include:
Molecular Degrader of Extracellular Proteins (MoDE) Platform: Harnessing a New Modality with Transformational Potential for the Treatment of Immunological and Inflammatory Disorders
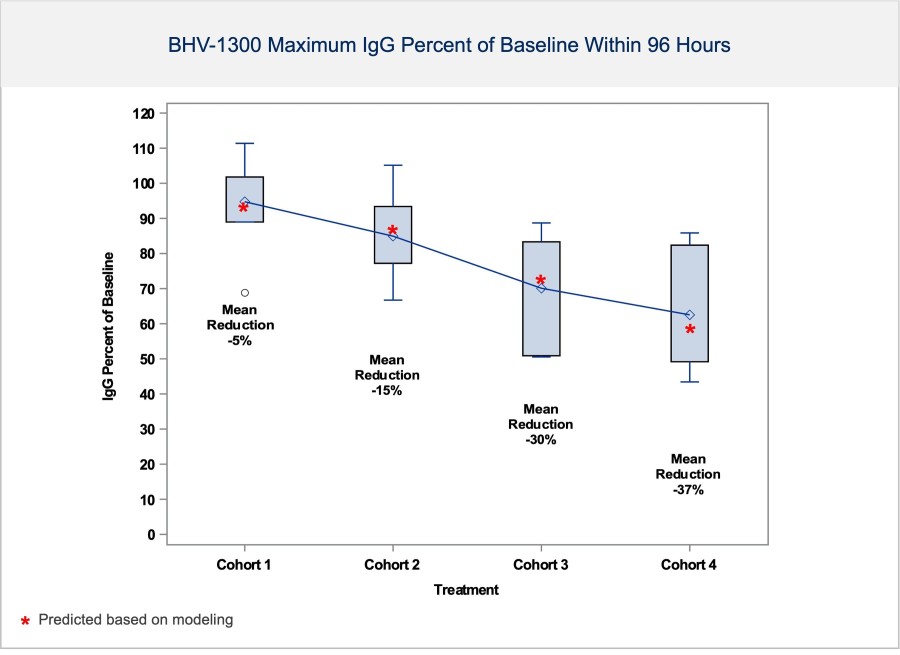
The Company unveiled new positive data from its ongoing Phase 1 single ascending dose (SAD) study with BHV-1300, a first-in-human IgG degrader that uses an ASGPR-bispecific from its MoDE platform. Emerging results in healthy subjects confirm that BHV-1300 rapidly and selectively lowers IgG in a dose-dependent manner in the first 4 cohorts completed to date (see Figure 1). Preliminary IgG lowering data is consistent with modeling, with dose- and time-dependent IgG lowering observed even in initial low-dose cohorts. Some subjects experienced IgG reductions as low as 50 to 70% of baseline. BHV-1300 demonstrated reduction of IgG without significantly impacting LFTs, albumin, LDL cholesterol or other serum labs. BHV-1300 has been safe and well tolerated to date, with no serious or severe adverse events. Most AEs were mild, deemed unrelated to study drug and resolved spontaneously. As expected from the selectivity of the molecule for IgG, when compared to placebo, there were no meaningful reductions in average IgA, IgM or IgE levels during the week after dosing. No adverse trends have been observed in vital signs or ECGs. Given the levels of IgG lowering observed to date, the company plans to evaluate approximately 6 cohorts of BHV-1300. Modeling suggests additional cohorts in the Phase 1 study will achieve > 70% lowering of IgG utilizing doses compatible with subcutaneous administration. Given the promising results of the SAD study thus far, the MAD study will proceed in patients with rheumatoid arthritis.
Ion Channel Platform: Forging Much-Needed Novel Treatments for Patients with Neurological and Neuropsychiatric Disorders
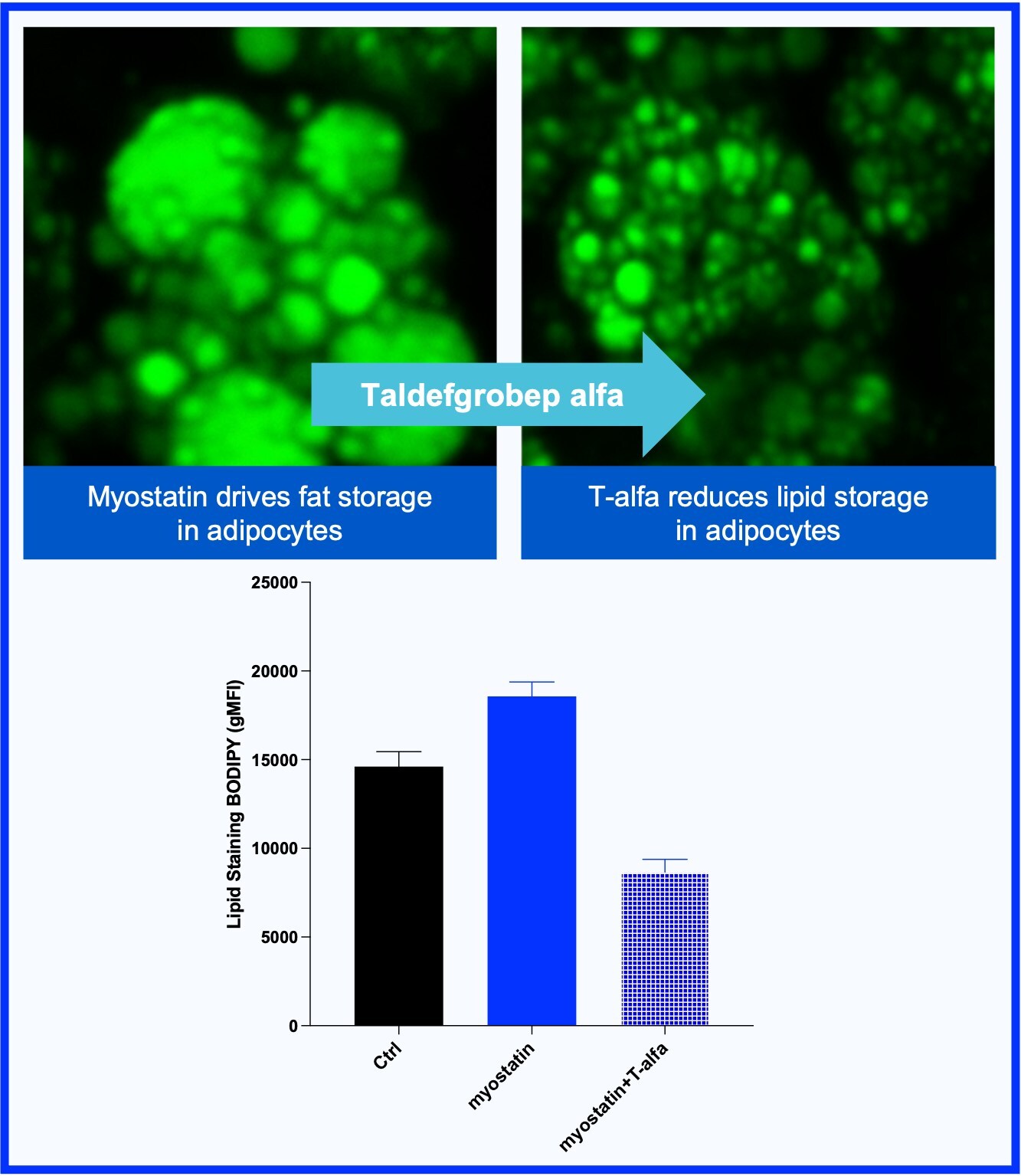
Myostatin Program: Advancing an Innovative Approach for Improving Muscle Health
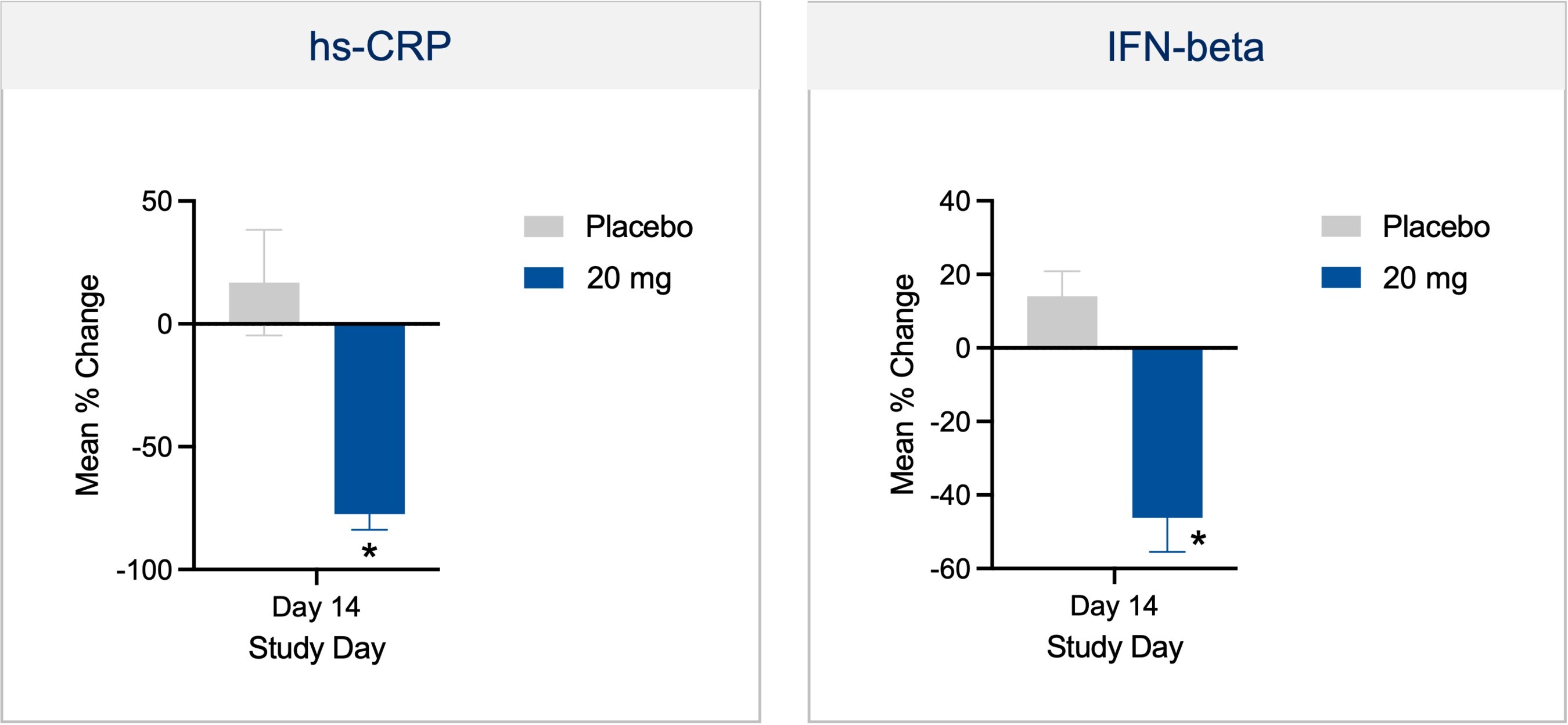
Neuroinflammation Platform: Selectively Targeting the Immune System to Treat Neurodegenerative Diseases
Glutamate Modulating Platform: Two Pivotal Trials in OCD and SCA Regulatory Workstreams Advance
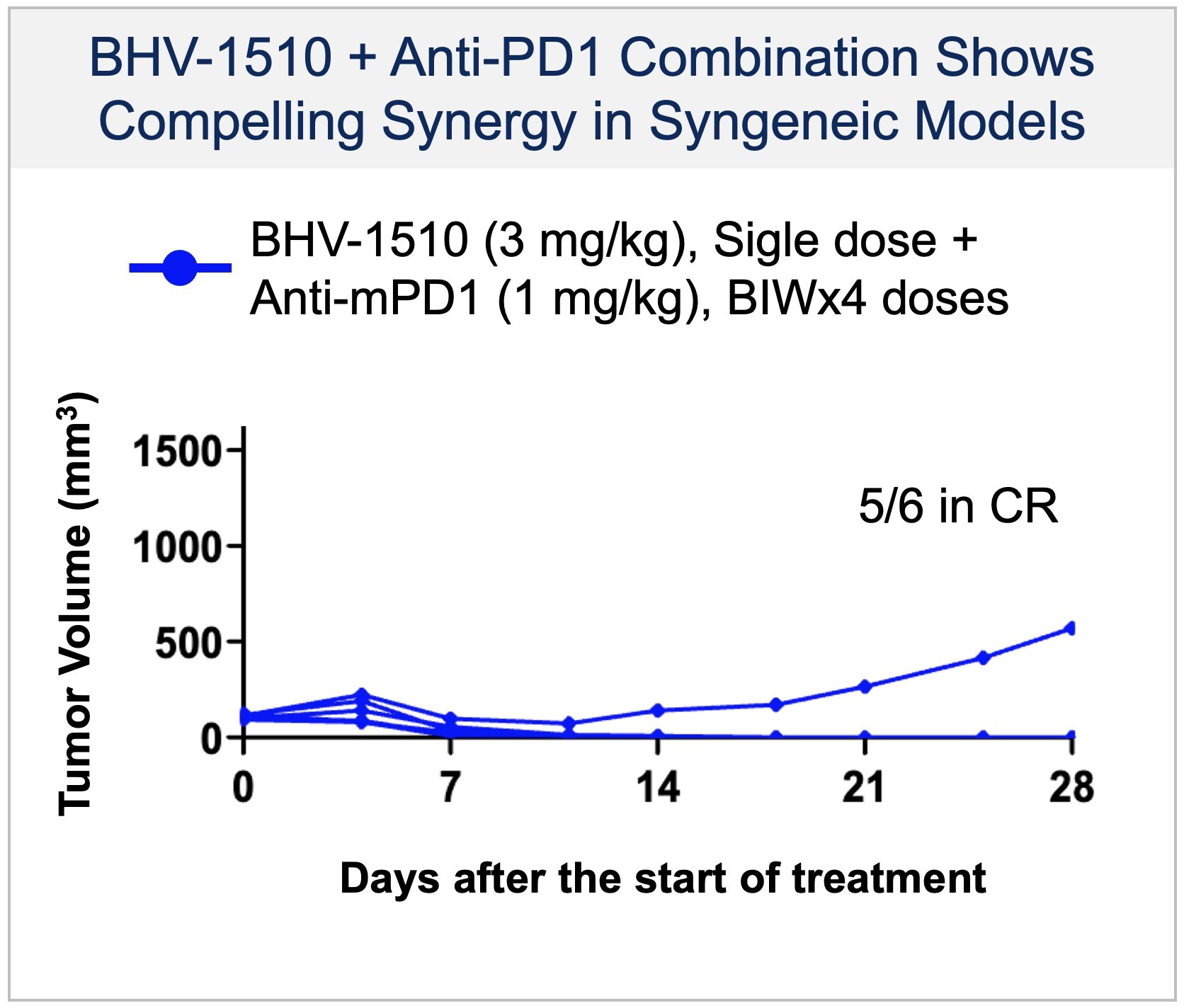
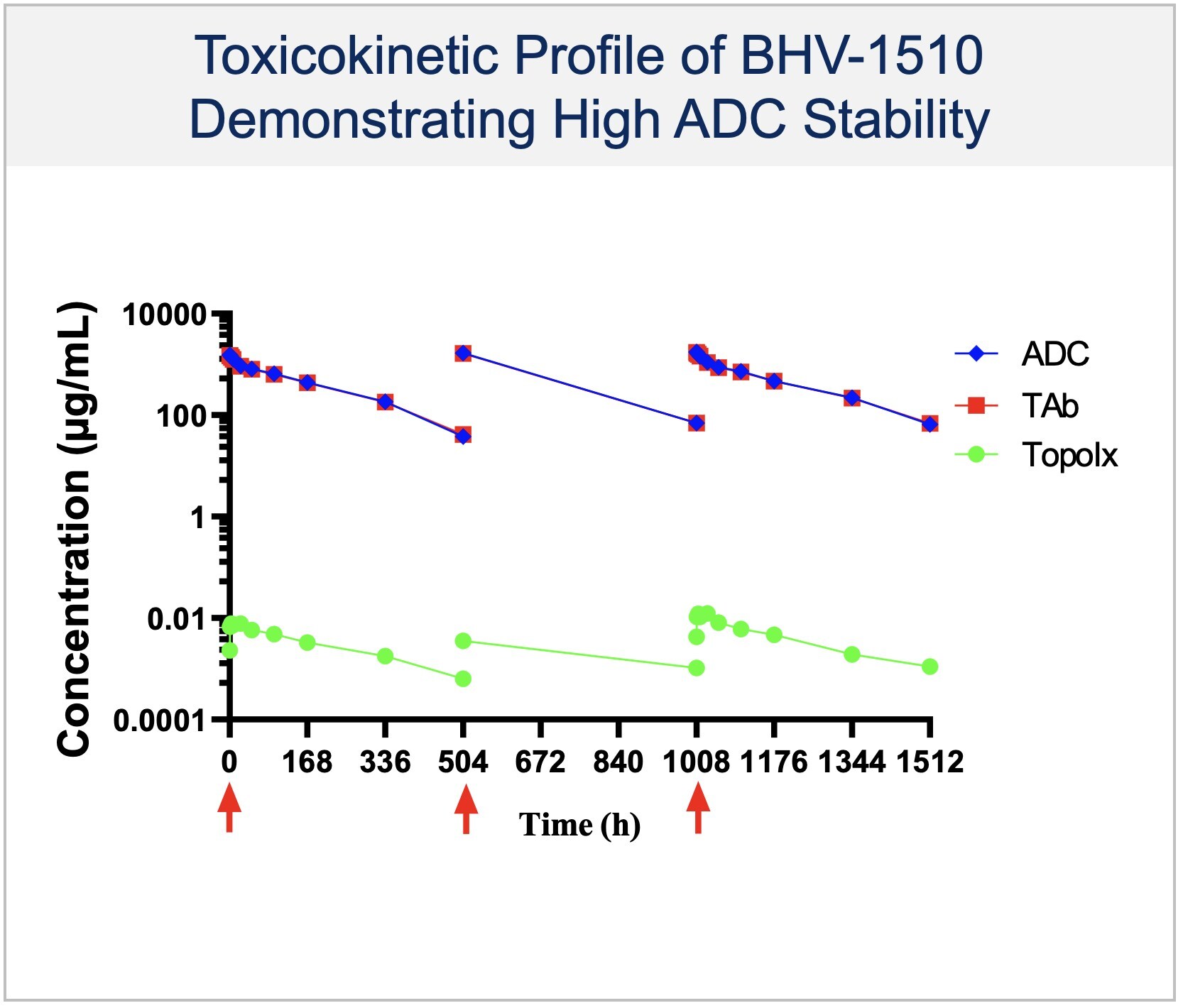
Oncology Platform: Building an Antibody Drug Conjugate (ADC) Franchise with Potential for Near- and Long-Term Value Creation
Vlad Coric, M.D., Chairman and Chief Executive Officer of Biohaven, commented on the Company's 2024 R&D Day: "Biohaven is leading the way in immune modulation with our first of its kind mechanism of action in MoDE degraders advancing through the early cohorts of Phase 1 testing. Equally important and exciting is that these data provide clinical validation for Biohaven's MoDE degrader platform, which represents an entirely novel class of drugs with rapid development timelines and unlimited clinical and commercial potential. The platform can efficiently generate compounds designed to selectively degrade a specific extracellular protein of interest, such as an individual disease-causing autoantibody. The advancement of BHV-1300 has accelerated the development of other assets from the MoDE platform. We anticipate delivering approximately ten clinical-stage degraders over the next three years with the goal of radically transforming the treatment of a broad range of diseases, including up to three additional compounds by the end of this year. This technology has the potential to transform the treatment of autoimmune disorders and disrupt current treatment paradigms across therapeutic areas."
"In addition to our MoDE platform, we are advancing novel science in multiple therapeutics areas including ion channel modulation for neurological and neuropsychiatric indications, myostatin and activin modulation for muscle health and obesity, TYK2/JAK1 inhibition for neuroinflammatory conditions, glutamate modulation in neuroscience and a new generation of ADCs in oncology," continued Dr. Coric. "I am so proud of the Biohaven team that is forging new scientific ground and working to improve the lives of patients not satisfied by current standard of care medications. Days matter for patients and their families, and the Biohaven team takes our responsibility seriously to efficiently move our programs forward to help those in need."
Bruce Car, DVM, Ph.D., DACVP, Chief Scientific Officer of Biohaven, commented, "We have built a high-performing team to tackle some of the most disabling diseases and conditions that face society. We are excited about the progress our R&D team is making in pursuing new druggable targets and disrupting older therapies with optimized technology with the goal of changing treatment paradigms. As we continue to advance our promising lead product candidates through upcoming milestones, our team will listen to the needs of patients and lean on our proven expertise in drug development execution to move with speed and efficiency on behalf of the millions of patients and families who are relying on our important work."
About Biohaven
Biohaven is a biopharmaceutical company focused on the discovery, development, and commercialization of life-changing treatments in key therapeutic areas, including immunology, neuroscience and oncology. The company is advancing its innovative portfolio of therapeutics, leveraging its proven drug development experience and multiple proprietary drug development platforms. Biohaven's extensive clinical and preclinical programs include Kv7 ion channel modulation for epilepsy and mood disorders; extracellular protein degradation for immunological diseases; TRPM3 antagonism for migraine and neuropathic pain; TYK2/JAK1 inhibition for neuroinflammatory disorders; glutamate modulation for OCD and SCA (spinocerebellar ataxia); myostatin inhibition for neuromuscular and metabolic diseases, including SMA and obesity; antibody recruiting bispecific molecules and antibody drug conjugates for cancer. For more information, visit www.biohaven.com.
Forward-looking Statements
This news release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. The use of certain words, including "continue", "plan", "will", "believe", "may", "expect", "anticipate" and similar expressions, is intended to identify forward-looking statements. Investors are cautioned that any forward-looking statements, including statements regarding the future development, timing and potential marketing approval and commercialization of development candidates, are not guarantees of future performance or results and involve substantial risks and uncertainties. Actual results, developments and events may differ materially from those in the forward-looking statements as a result of various factors including: the expected timing, commencement and outcomes of Biohaven's planned and ongoing clinical trials; the timing of planned interactions and filings with the FDA; the timing and outcome of expected regulatory filings; complying with applicable U.S. regulatory requirements; the potential commercialization of Biohaven's product candidates; the potential for Biohaven's product candidates to be first in class therapies; and the effectiveness and safety of Biohaven's product candidates. Additional important factors to be considered in connection with forward-looking statements are described in Biohaven's filings with the Securities and Exchange Commission, including within the sections titled "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations". The forward-looking statements are made as of the date of this news release, and Biohaven does not undertake any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
MoDE and MATE are trademarks of Biohaven Therapeutics Ltd.
Libtayo is a registered trademark of Regeneron Pharmaceuticals, Inc.
Investor Contact:
Jennifer Porcelli
Vice President, Investor Relations
This email address is being protected from spambots. You need JavaScript enabled to view it.
201-248-0741
Media Contact:
Mike Beyer
Sam Brown Inc.
This email address is being protected from spambots. You need JavaScript enabled to view it.
312-961-2502

| Last Trade: | US$36.42 |
| Daily Change: | 0.67 1.87 |
| Daily Volume: | 657,289 |
| Market Cap: | US$3.680B |
November 25, 2024 November 12, 2024 October 02, 2024 | |

Cue Biopharma is developing the first-ever class of therapeutics for the treatment of cancer that mimic the natural signals, or “Cues”, of the immune system. This novel class of injectable biologics selectively engages and modulates tumor-specific T cells directly within the patient’s body to transform...
CLICK TO LEARN MORE
Astria Therapeutics is a biopharmaceutical company, and our mission is to bring life-changing therapies to patients and families affected by rare and niche allergic and immunological diseases. Our lead program, STAR-0215, is a monoclonal antibody inhibitor of plasma kallikrein in clinical development...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB