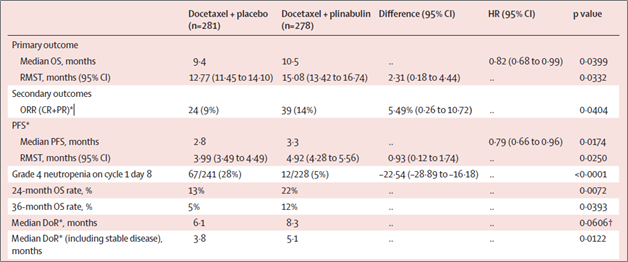
FLORHAM PARK, N.J., Nov. 11, 2024 (GLOBE NEWSWIRE) -- BeyondSpring Inc. (NASDAQ: BYSI) (“BeyondSpring” or the “Company”), a clinical-stage global biopharmaceutical company developing innovative cancer therapies, today announced that phase 2 IIT (Investigator-initiated) data on the first 30 patients dosed with plinabulin in the 303 Study of patients with non-small cell lung cancer (NSCLC) after disease progression on... Read More