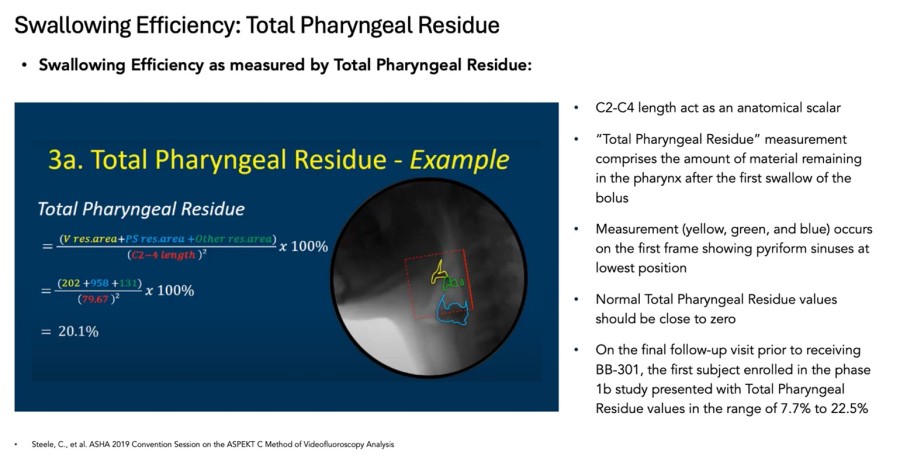
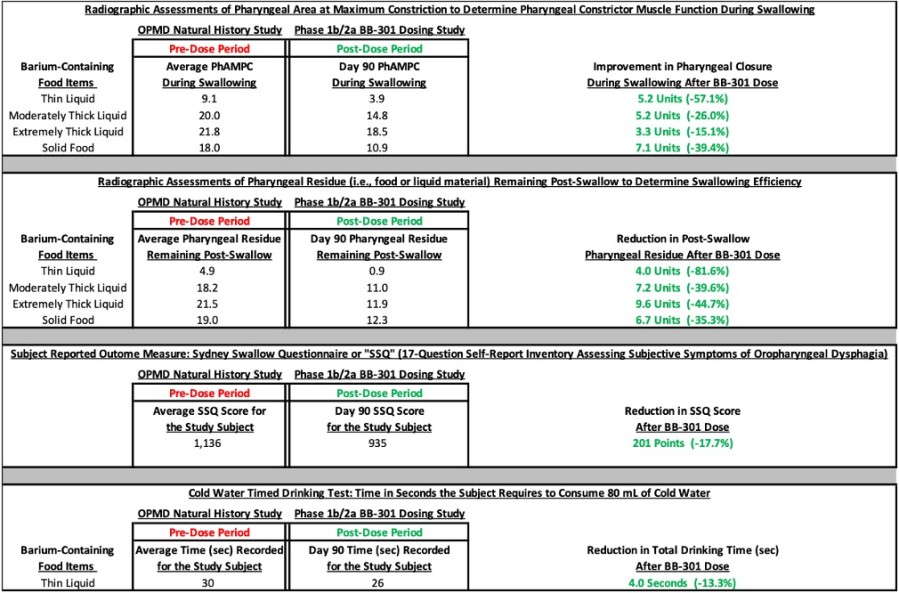
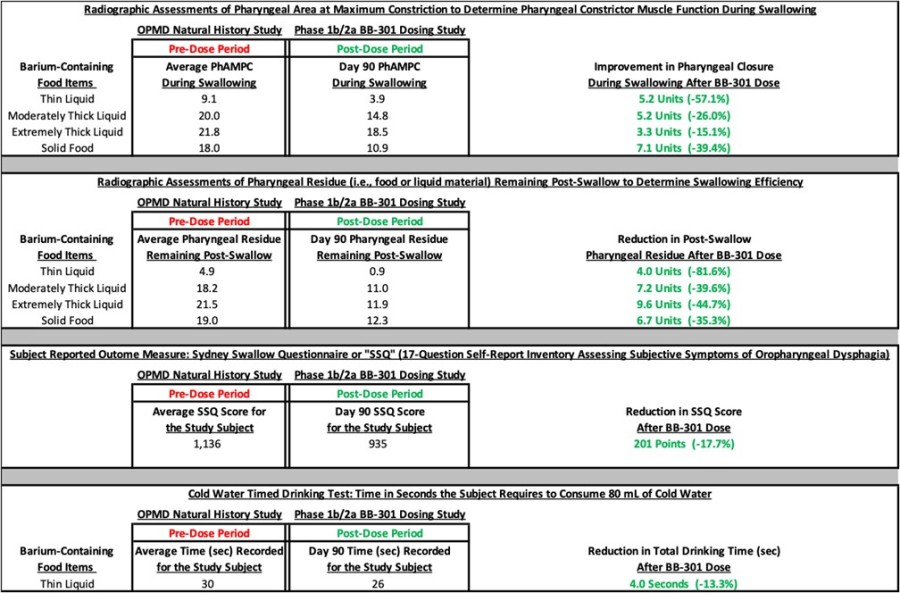
HAYWARD, Calif., Nov. 26, 2024 (GLOBE NEWSWIRE) -- Benitec Biopharma Inc. (NASDAQ: BNTC) (“Benitec” or the “Company”), a clinical-stage, gene therapy-focused, biotechnology company developing novel genetic medicines based on its proprietary “Silence and Replace” DNA-directed RNA interference ("ddRNAi") platform, today announced that its management team will participate in the following upcoming investor conferences. Piper... Read More