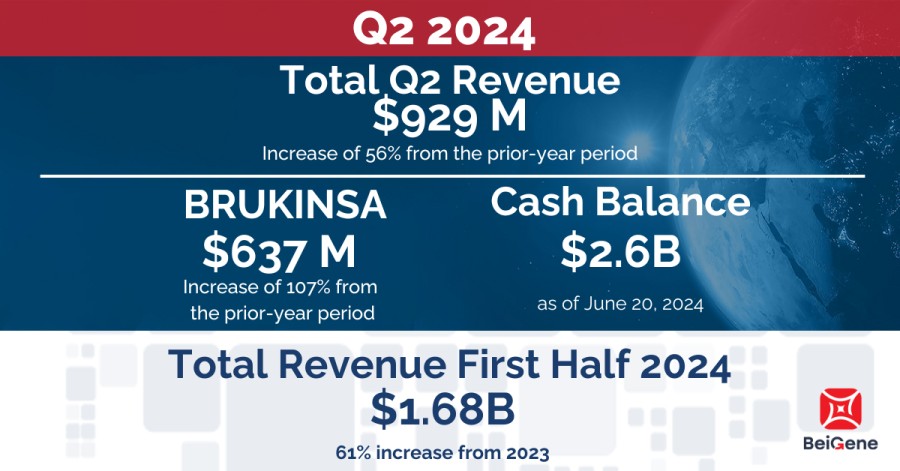
SAN MATEO, Calif. / Dec 12, 2024 / Business Wire / BeiGene, Ltd. (NASDAQ: BGNE; HKEX: 06160; SSE: 688235), a global oncology company that intends to change its name to BeOne Medicines Ltd., today announced it has entered into a global licensing agreement with CSPC Zhongqi Pharmaceutical Technology (Shijiazhuang) Co., Ltd. (“CSPC”) for SYH2039, a novel methionine adenosyltransferase 2A (MAT2A)-inhibitor being explored for... Read More