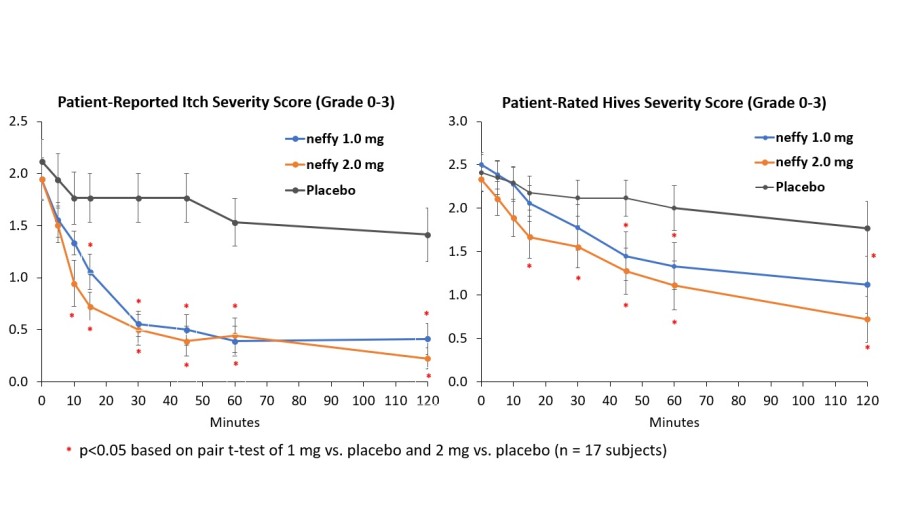
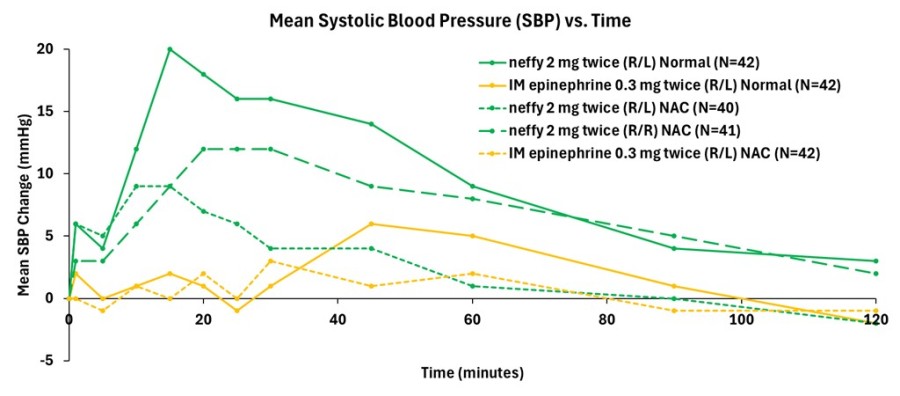
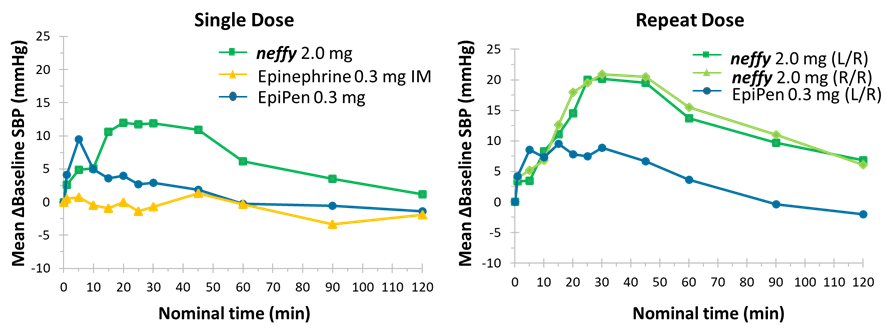
Inclusion of neffy on Express Scripts commercial formularies was effective November 22, 2024 and expands access to patients and caregivers managing Type 1 Allergic Reactions SAN DIEGO, Dec. 19, 2024 (GLOBE NEWSWIRE) -- ARS Pharmaceuticals, Inc. (Nasdaq: SPRY), a biopharmaceutical company dedicated to empowering at-risk patients and caregivers to better protect themselves from allergic reactions that could lead to... Read More