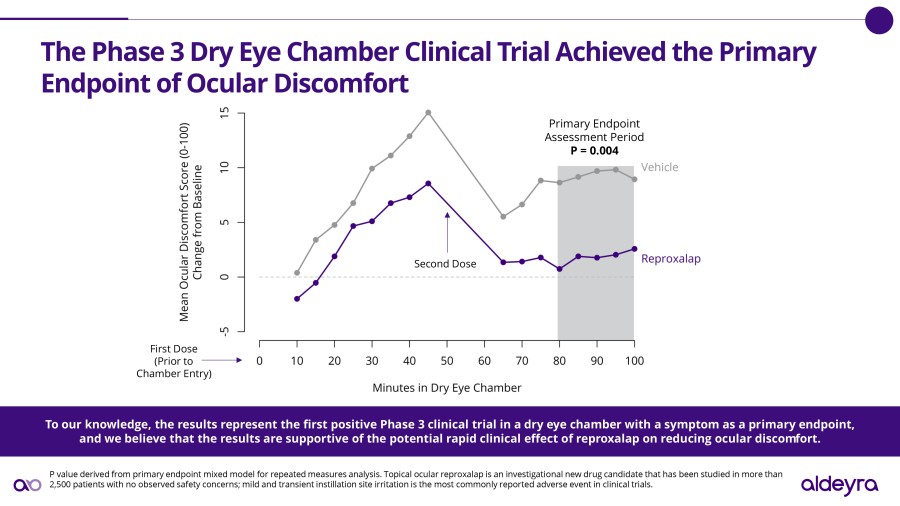
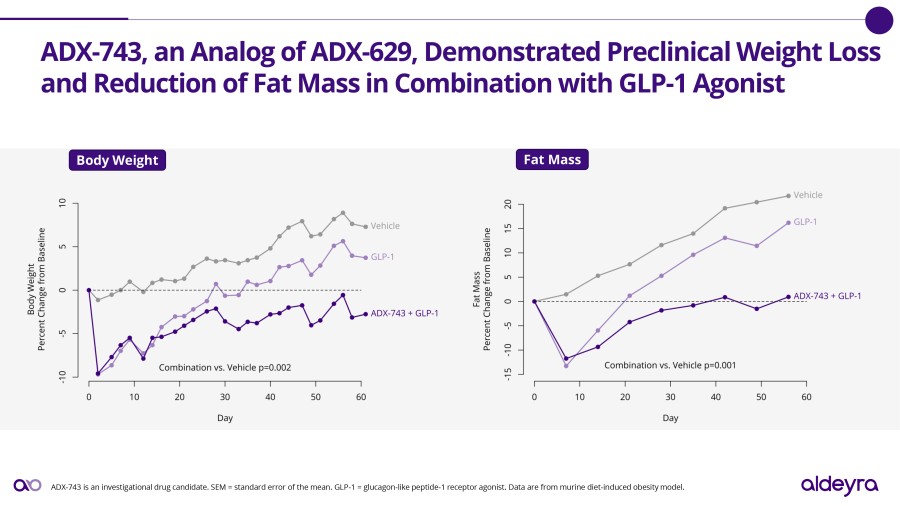
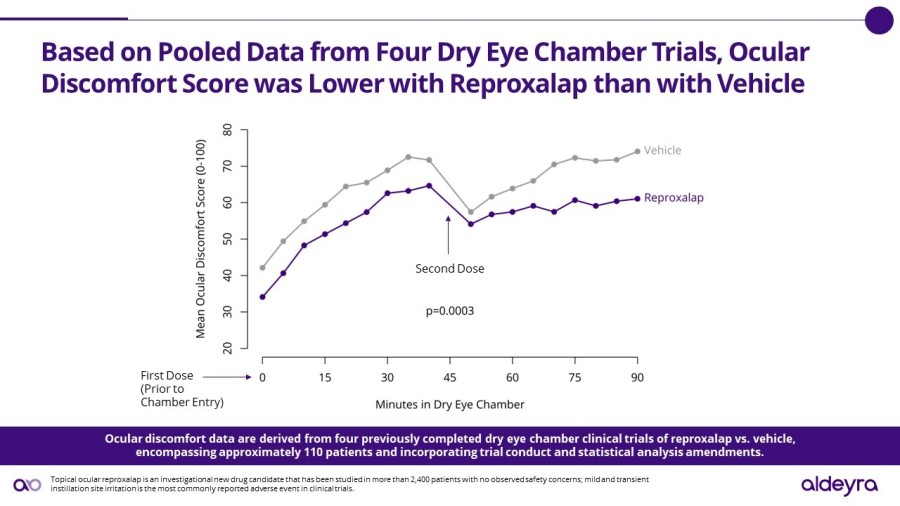
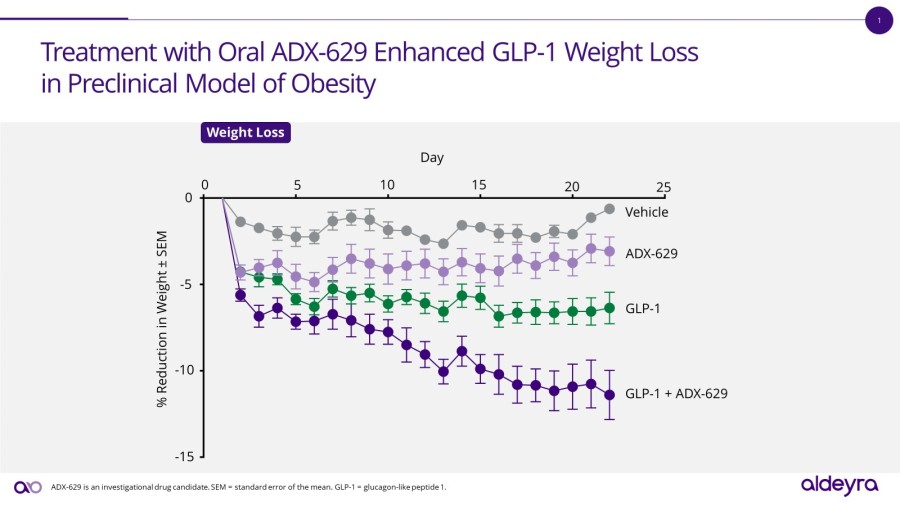
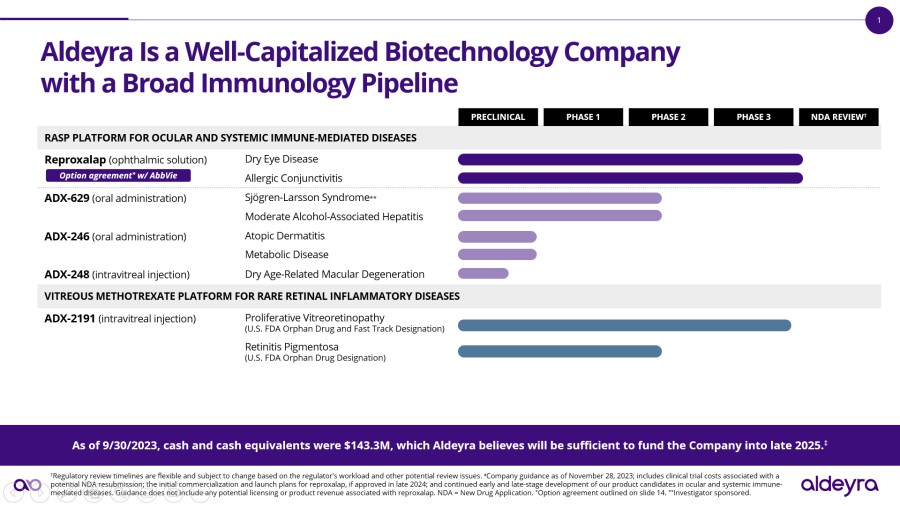
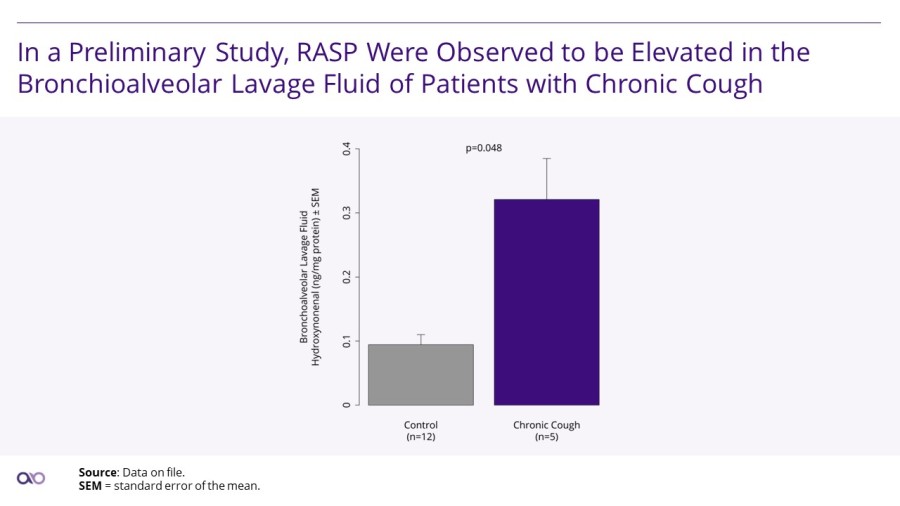
LEXINGTON, Mass. / Dec 02, 2024 / Business Wire / Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated and metabolic diseases, today announced that Todd C. Brady, M.D., Ph.D., President and Chief Executive Officer, will participate in the Eyes Wide Open on Ophthalmology Panel at the Citi 2024 Global Healthcare... Read More