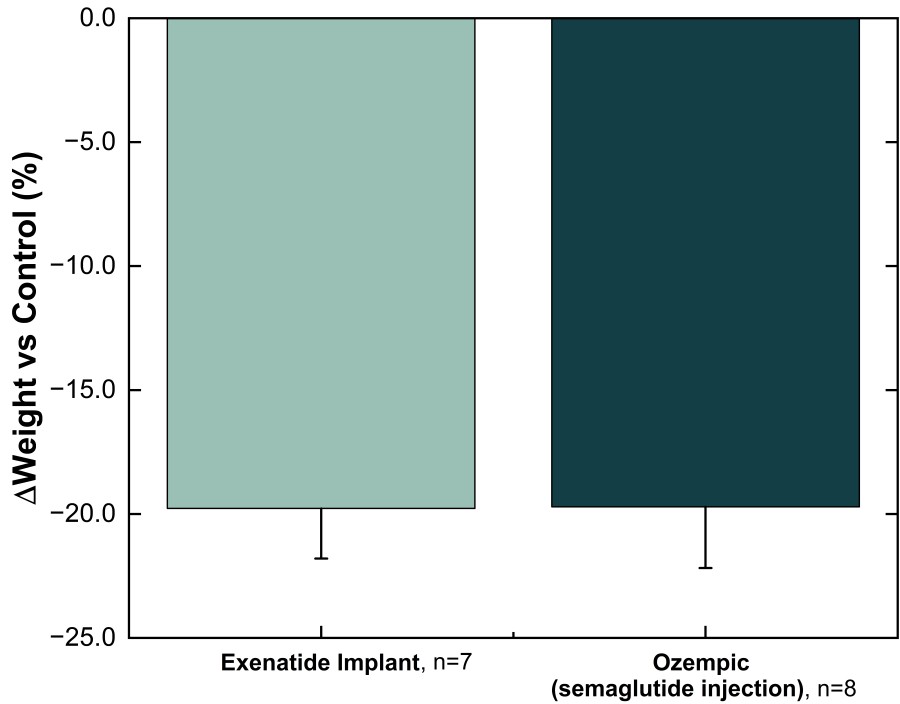
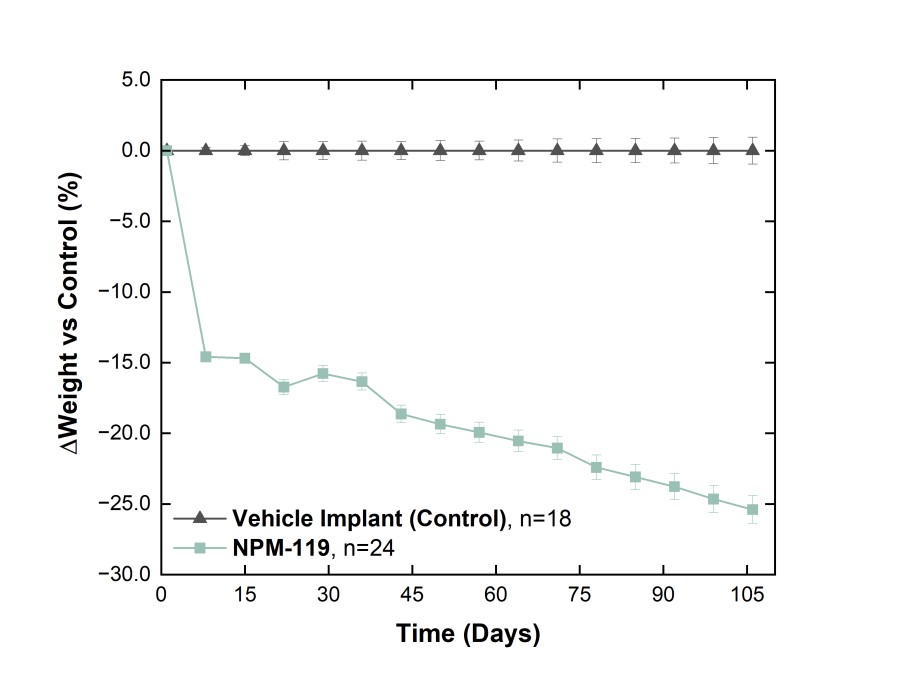
NPM-115 clinical program utilizes a miniature, GLP-1 (exenatide) implant designed to provide comparable efficacy to semaglutide, with twice-yearly administration Study represents the first clinical application of NanoPortal™, the Company’s proprietary drug implant platform technology ALAMEDA, Calif. / Dec 19, 2024 / Business Wire / Vivani Medical, Inc. (Nasdaq: VANI) (“Vivani” or the “Company”), an innovative... Read More