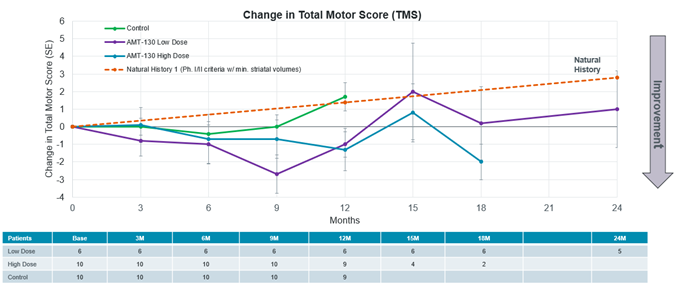
U.S. Food and Drug Administration (FDA) agrees that data from ongoing Phase I/II studies compared to a natural history external control may serve as the primary basis for a Biologics License Application (BLA) for Accelerated Approval FDA agrees that the composite Unified Huntington’s Disease Rating Scale (cUHDRS) may serve as an intermediate clinical endpoint for Accelerated Approval ~ Conference call today at 8:30 a.m. ET... Read More