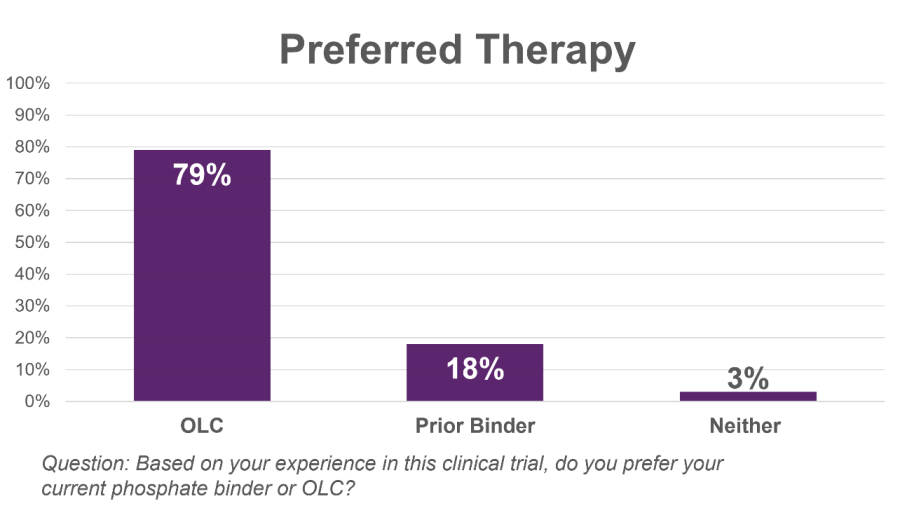
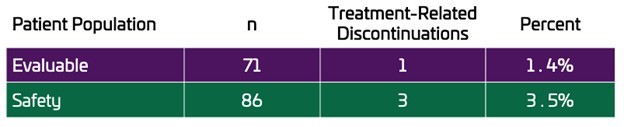
LOS ALTOS, Calif., Dec. 17, 2024 (GLOBE NEWSWIRE) -- Unicycive Therapeutics, Inc. (Nasdaq: UNCY), a clinical-stage biotechnology company developing therapies for patients with kidney disease (the “Company” or “Unicycive”), today announced that data from the Company’s oxylanthanum carbonate (OLC) bioequivalence study in healthy volunteers was published in the peer-reviewed journal, Clinical Therapeutics . The publication,... Read More