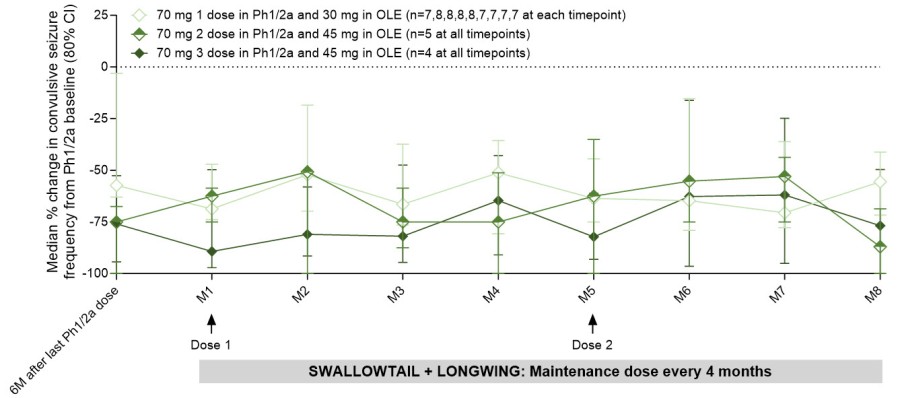
Substantial and durable reductions in convulsive seizure frequency observed in patients treated with 2 or 3 doses of 70mg followed by 45mg maintenance dosing on top of the best available anti-seizure medicines Patients experienced continuous improvements in multiple measures of cognition and behavior with ongoing treatment... Read More