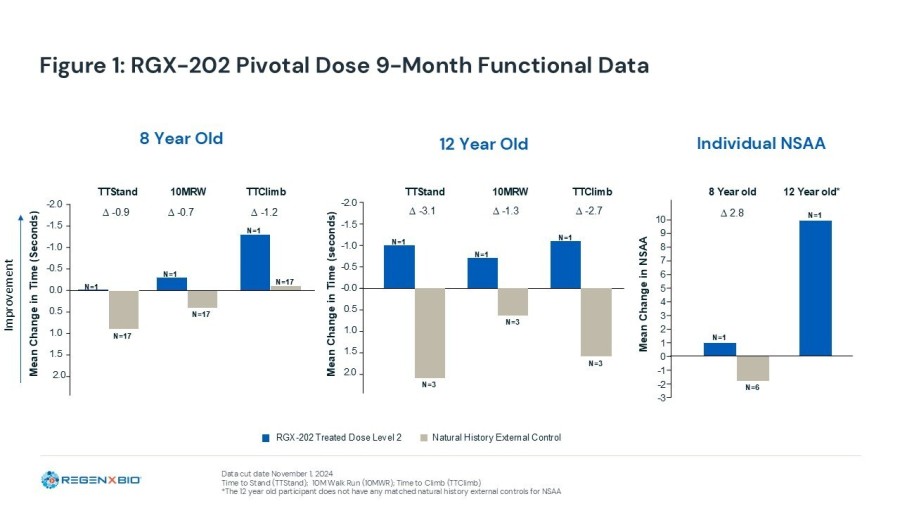
Alignment achieved with FDA on AFFINITY DUCHENNE ® pivotal program and access to accelerated approval; BLA expected in 2026 Pivotal trial of RGX-202 is enrolling ambulatory patients aged 1 and above with first patient dosed Phase I/II data show RGX-202 recipients exceeding external natural history and established benchmarks... Read More