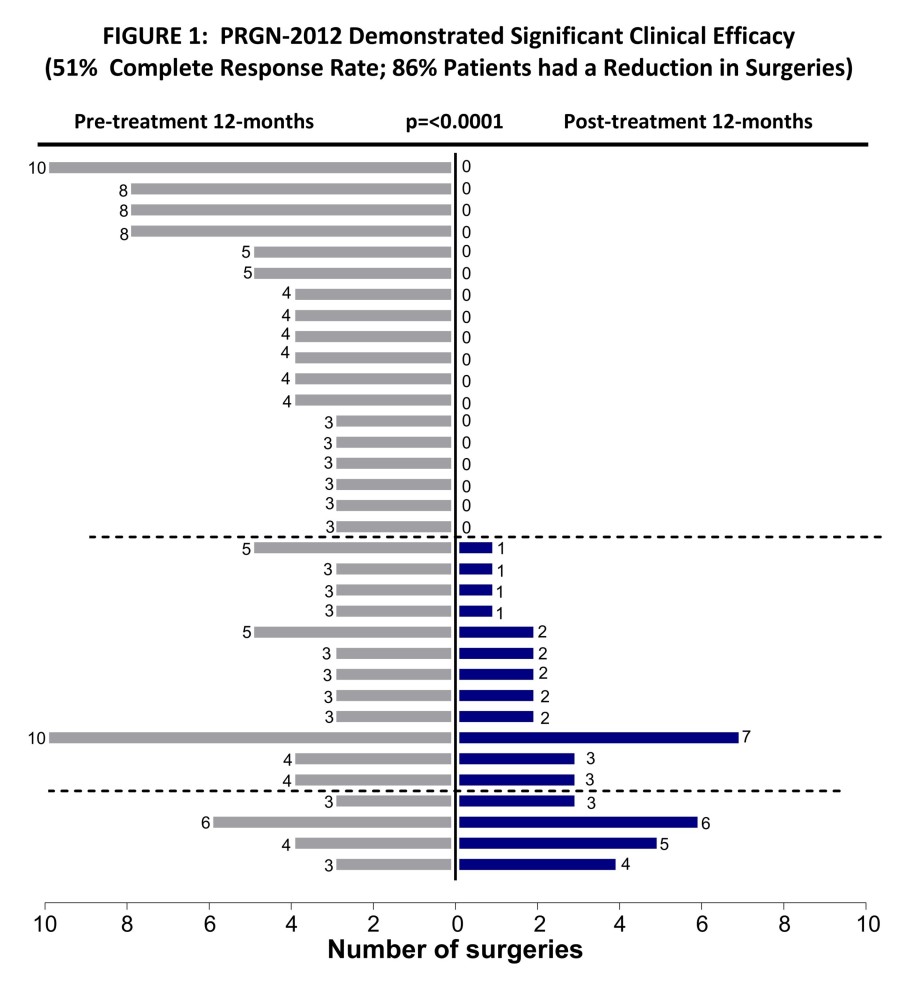
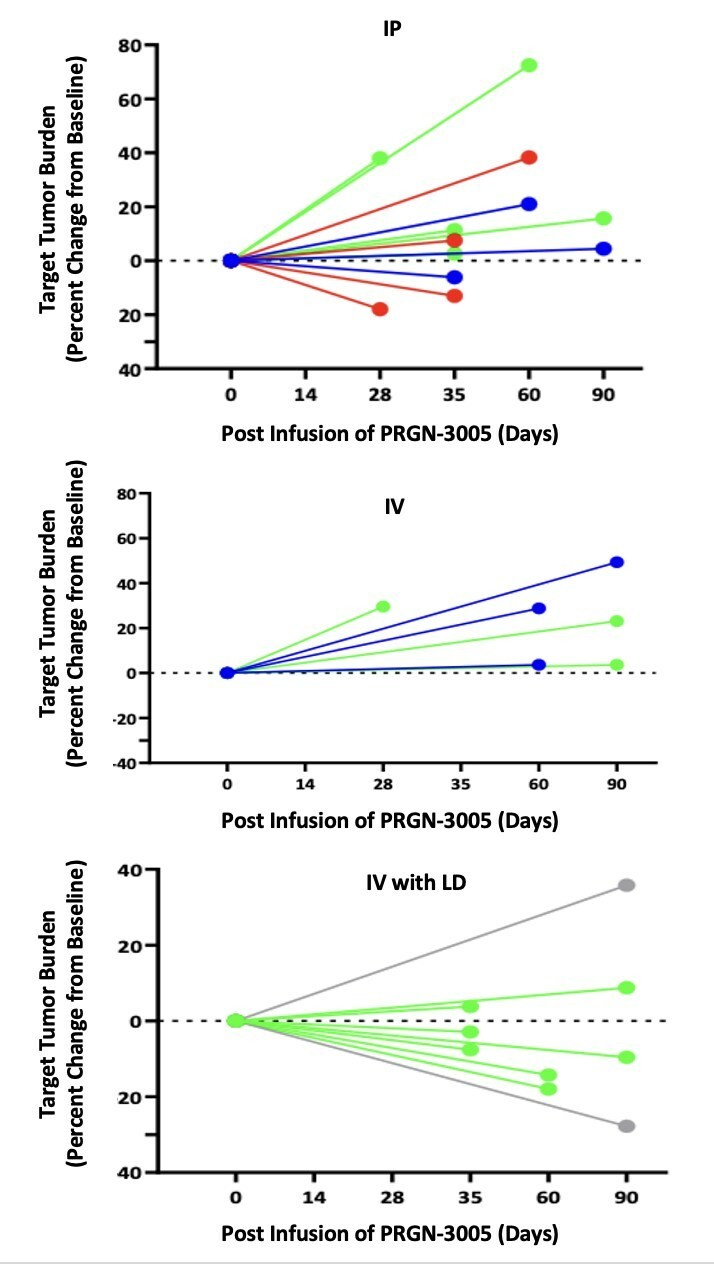
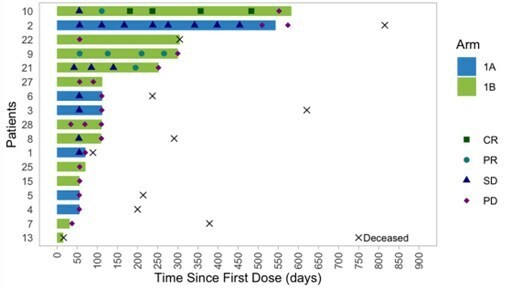
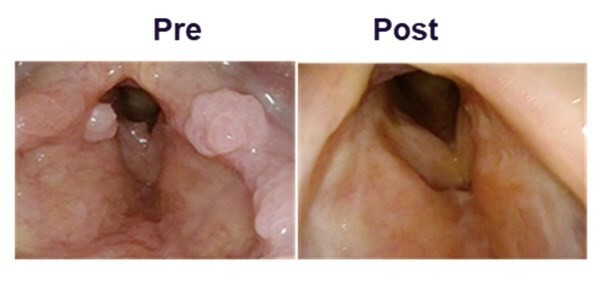
Completed pre-BLA meeting with FDA with full alignment on content of BLA, including CMC module, and path for fourth quarter 2024 rolling BLA submission for PRGN-2012 † in RRP under accelerated approval pathway Commercial and manufacturing readiness campaign underway for PRGN-2012 in anticipation of a potential 2025 launch Confirmatory clinical trial for PRGN-2012 in RRP was initiated in accordance with guidance from FDA to... Read More