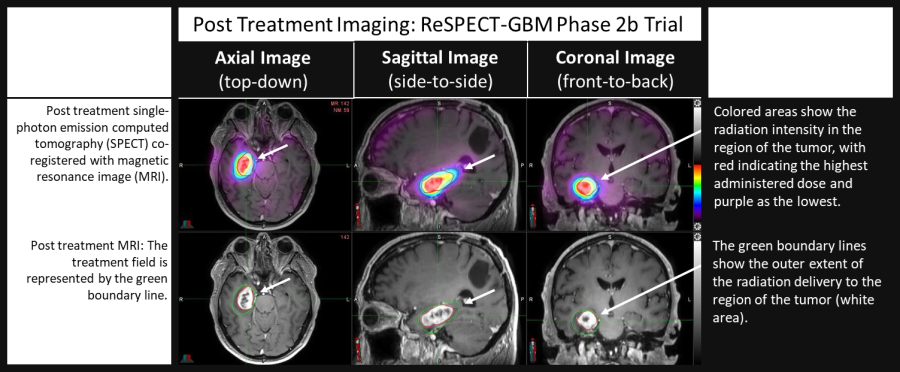
Single intrathecal dose of Rhenium ( 186 Re) Obisbemeda delivers favorable responses in cerebrospinal fluid circulating tumor cell count, imaging, and clinical evaluation Both single-dose and multiple-dose expansion trials planned in 2025 AUSTIN, Texas, Dec. 17, 2024 (GLOBE NEWSWIRE) -- Plus Therapeutics, Inc. (Nasdaq: PSTV) (“Plus” or the “Company”), a clinical-stage pharmaceutical company developing targeted... Read More