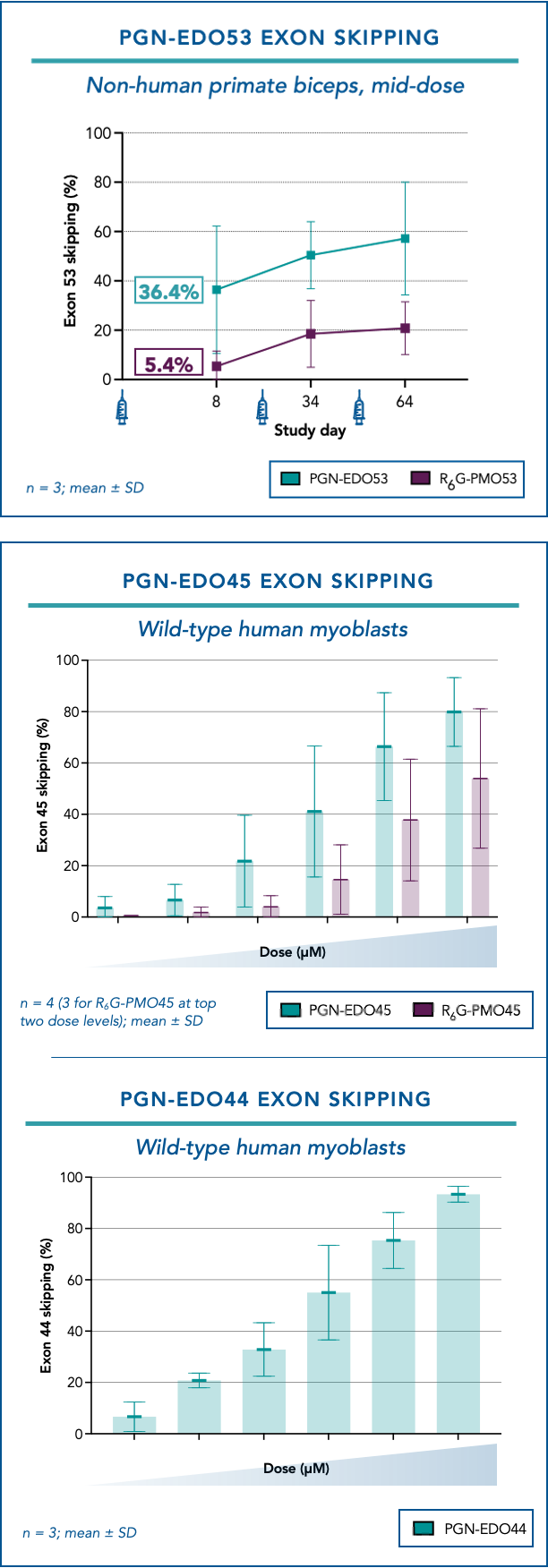
Company continues to advance PGN-EDO51 in CONNECT1-EDO51, with the 10 mg/kg cohort now fully enrolled BOSTON / Dec 16, 2024 / Business Wire / PepGen Inc . (Nasdaq: PEPG), a clinical-stage biotechnology company advancing the next generation of oligonucleotide therapies with the goal of transforming the treatment of severe neuromuscular and neurological diseases, today announced that the Company received a clinical hold notice... Read More