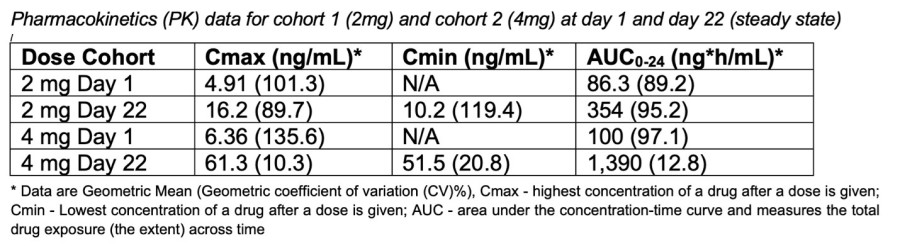
SRC recommended that the trial escalate to the next dose level of 15mg capsule No dose-limiting toxicities (DLTs) observed to date No rash observed to date MIAMI, Nov. 20, 2024 (GLOBE NEWSWIRE) -- Pasithea Therapeutics Corp. (NASDAQ: KTTA) (“Pasithea” or the “Company”), a clinical-stage biotechnology company developing PAS-004, a next-generation macrocyclic MEK inhibitor, for the treatment of neurofibromatosis type 1 (NF1)... Read More