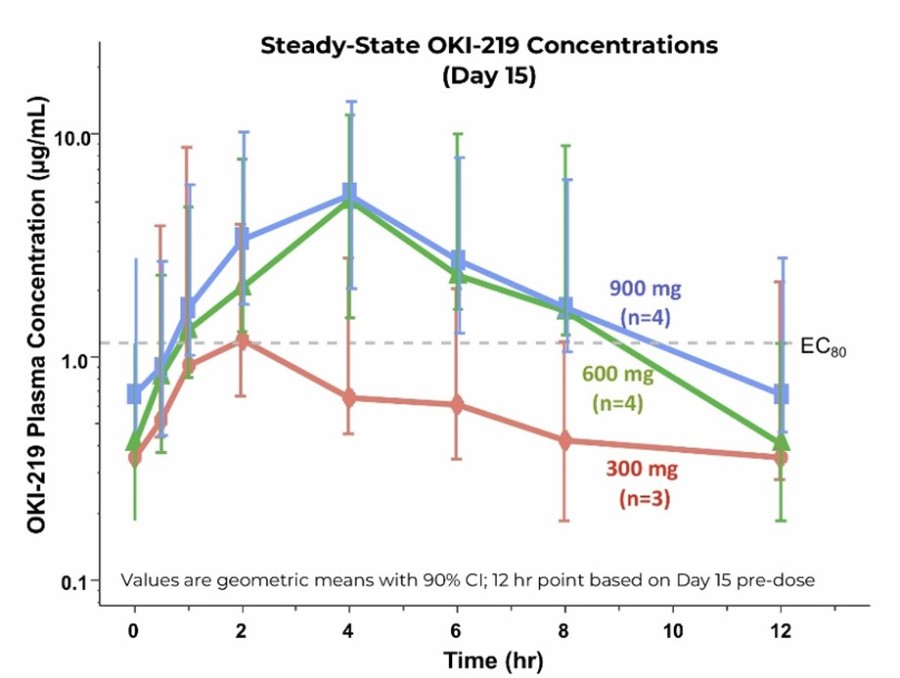
OKI-219 is well-tolerated across all doses, and no dose interruptions, delays, reductions, or discontinuations were reported Initial patient data show exposures of OKI-219 exceeding levels associated with robust antitumor activity in preclinical models Data support the initiation of Part 1b of PIKture-01 evaluating OKI-219 in... Read More