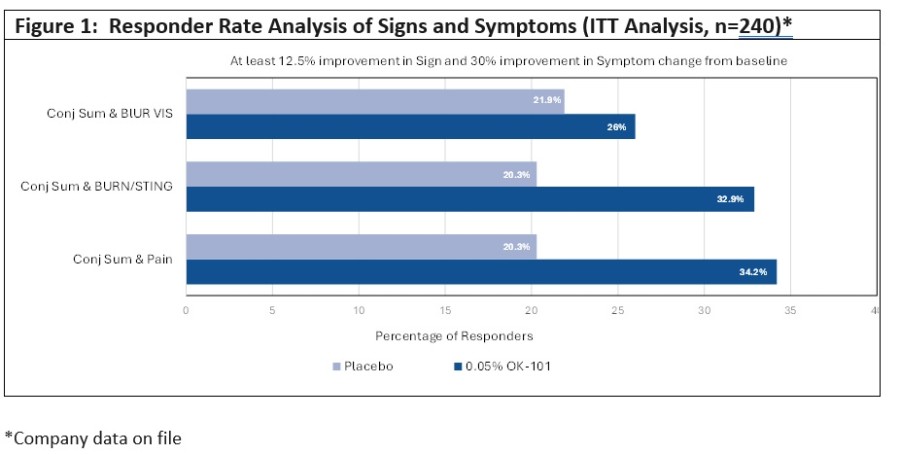
LONDON and NEW YORK, Nov. 26, 2024 (GLOBE NEWSWIRE) -- OKYO Pharma Limited (NASDAQ: OKYO), a clinical-stage biopharmaceutical company developing innovative therapies for the treatment of neuropathic corneal pain (NCP), a severe ocular condition without an FDA approved therapy, and for inflammatory dry eye disease (DED), a multi-billion-dollar market, is pleased to announce that its CEO, Gary S. Jacob, Ph.D., will be... Read More