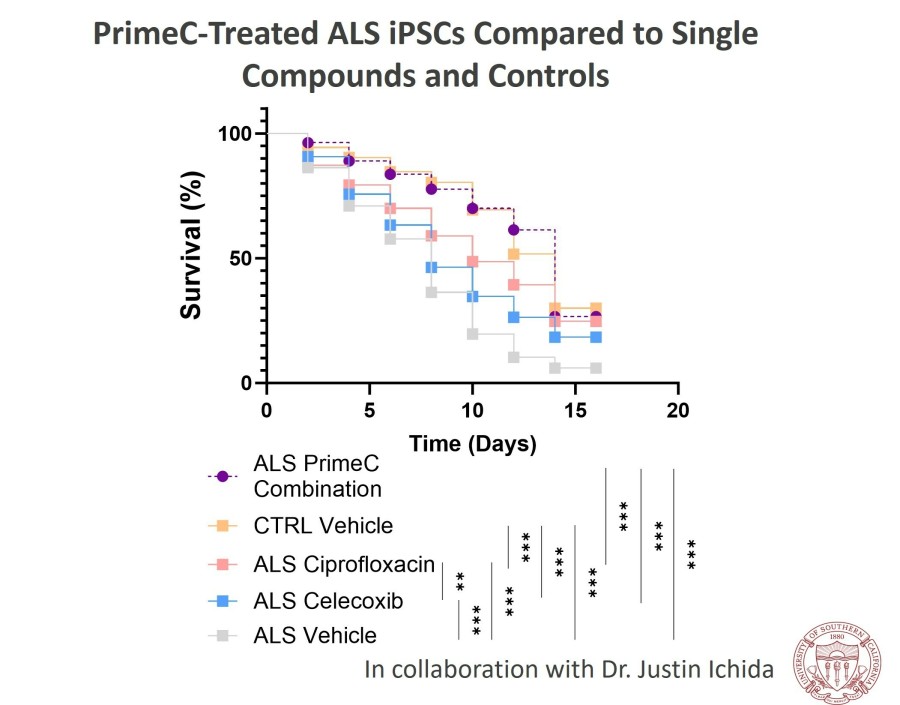
CAMBRIDGE, Mass. , Dec. 18, 2024 /PRNewswire/ -- NeuroSense Therapeutics Ltd. (Nasdaq: NRSN) ("NeuroSense"), a late-stage clinical biotechnology company developing novel treatments for severe neurodegenerative diseases, today provided business update with corporate highlights to date and third quarter financial results. "The completion of the 18-month Phase 2b PARADIGM study was a major milestone for NeuroSense. The results... Read More