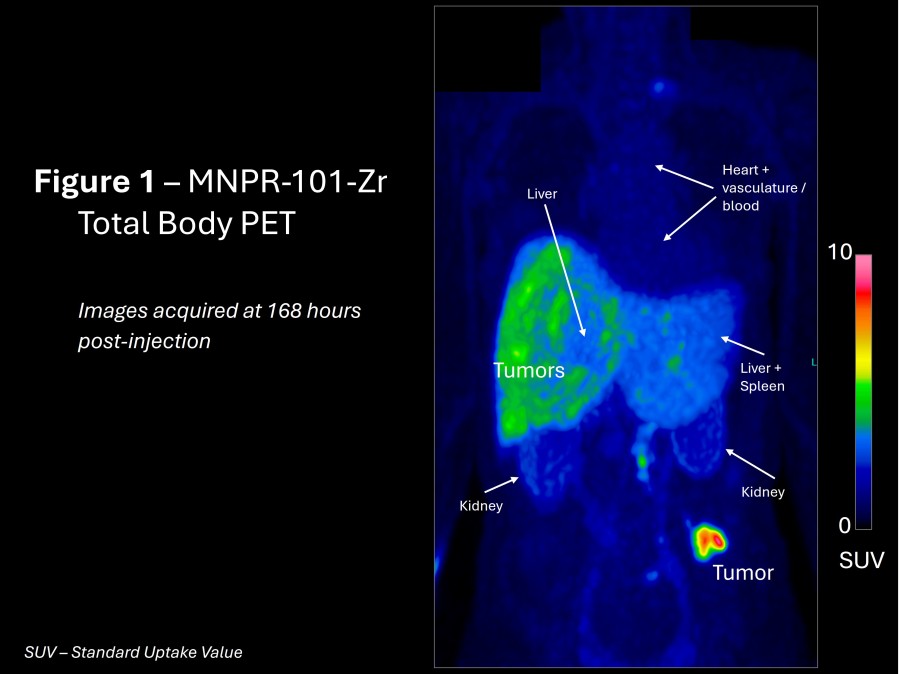
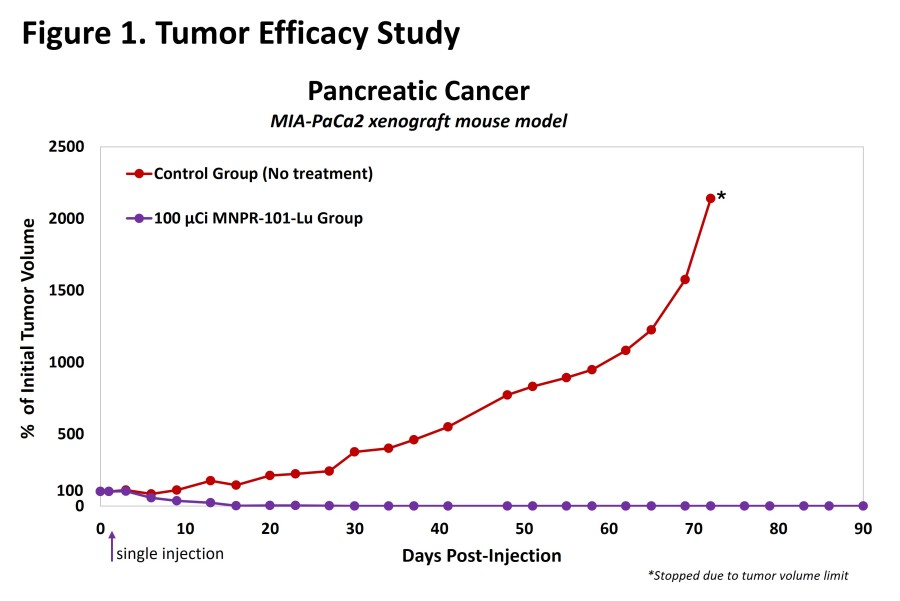
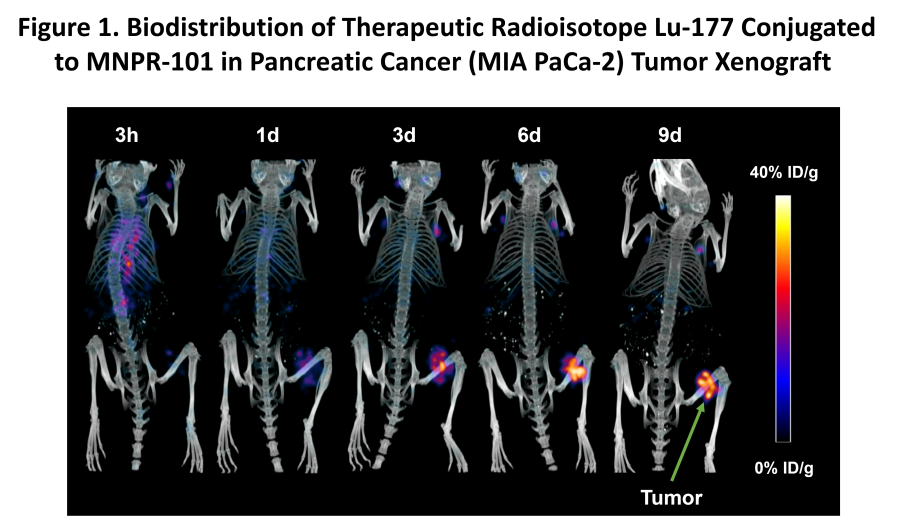
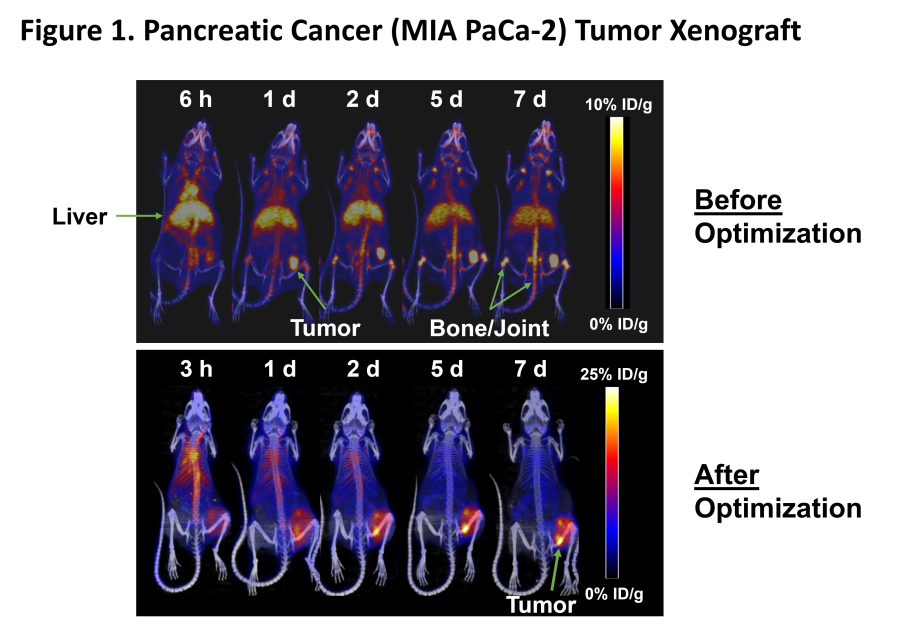
WILMETTE, Ill., Dec. 20, 2024 (GLOBE NEWSWIRE) -- Monopar Therapeutics Inc. (Nasdaq: MNPR) (“Monopar” or the “Company”) , a clinical-stage biotechnology company focused on developing innovative treatments for patients with unmet medical needs, today announced the pricing of an underwritten registered offering of 798,655 shares of its common stock at an offering price of $23.79 per share. In addition to the shares sold in the... Read More