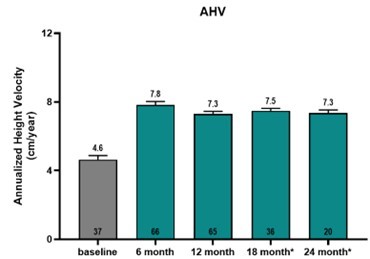
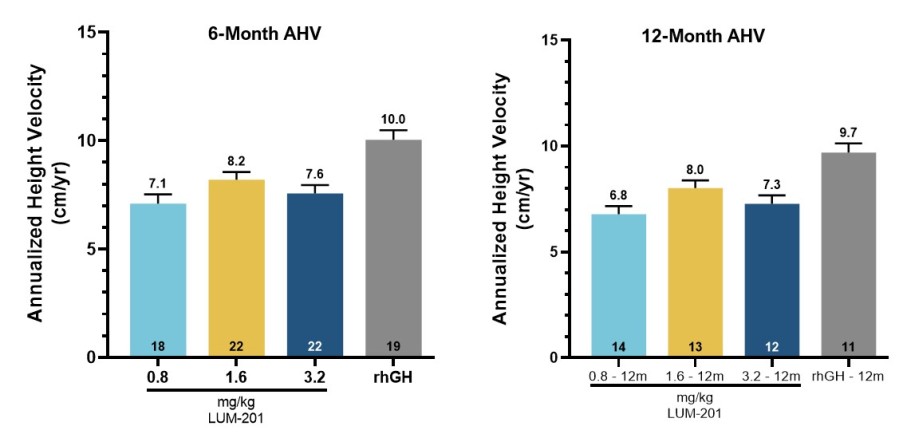
AUSTIN, Texas and GREENWICH, Conn., Dec. 12, 2024 (GLOBE NEWSWIRE) -- Lumos Pharma, Inc. (NASDAQ:LUMO) (“Lumos Pharma” or the “Company”), a clinical stage biopharmaceutical company focused on therapeutics for rare diseases, and Double Point Ventures LLC (“DPV”) today announced the successful completion of the previously announced tender offer for Lumos Pharma’s shares at a purchase price of (i) $4.25 per share in cash at... Read More