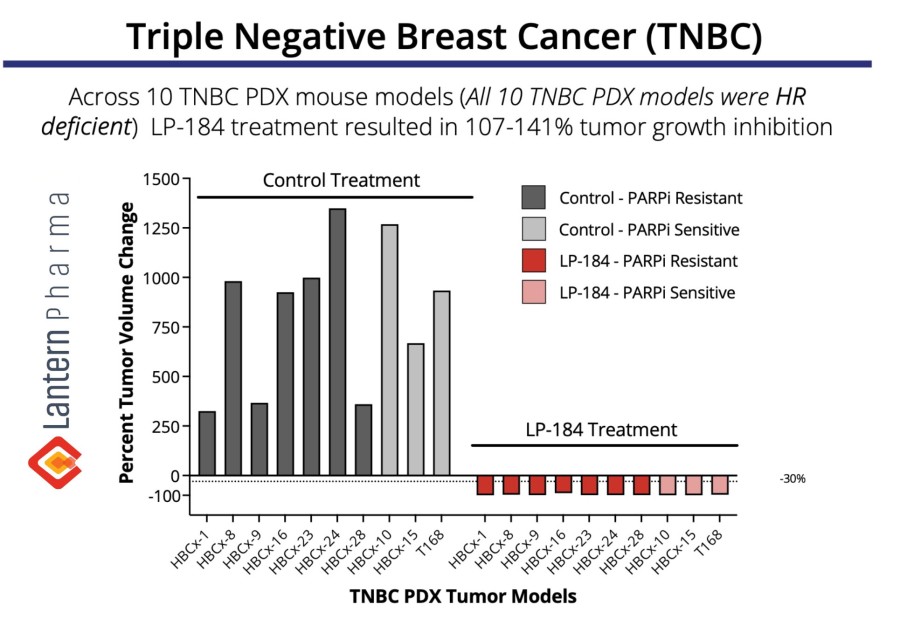
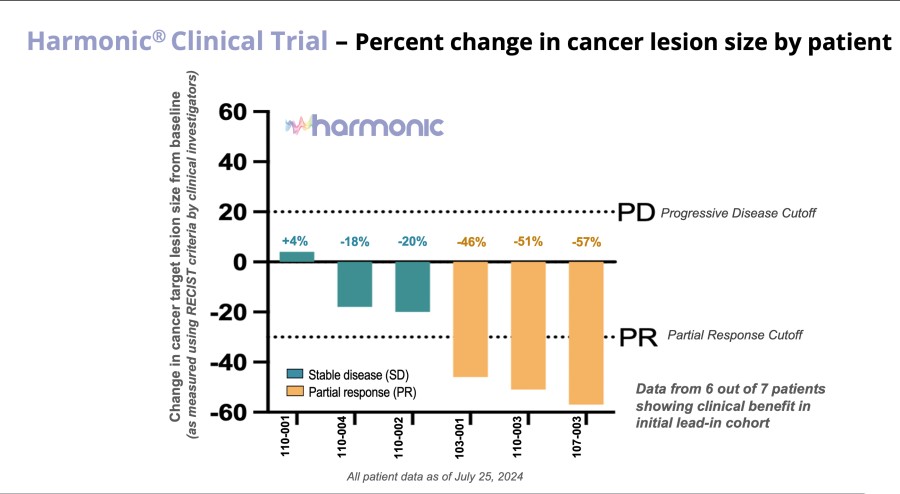
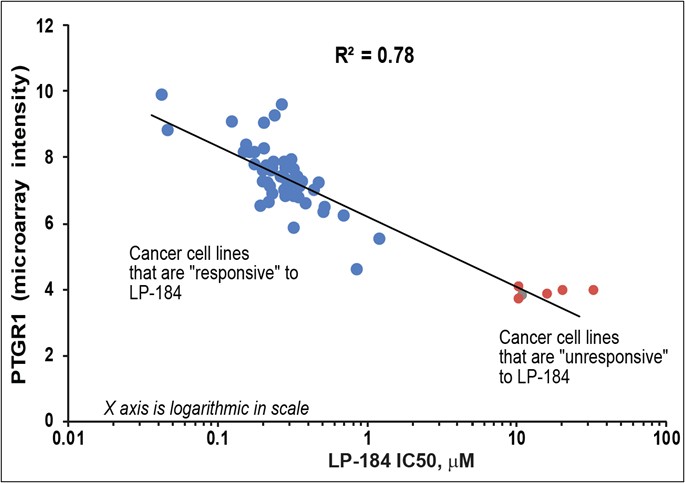
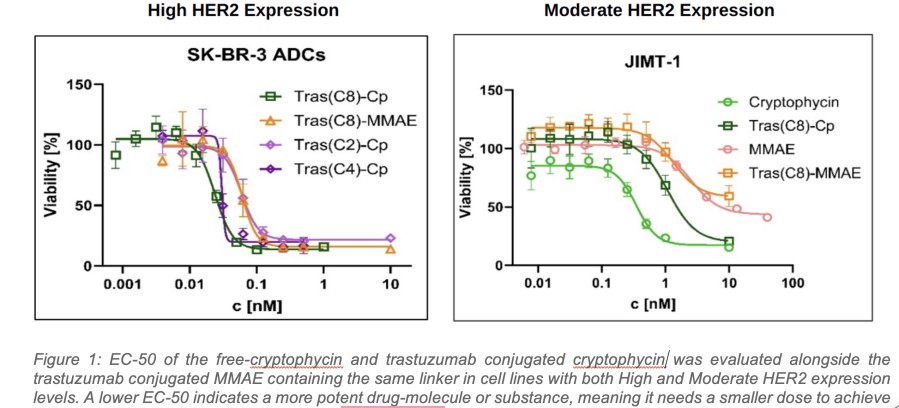
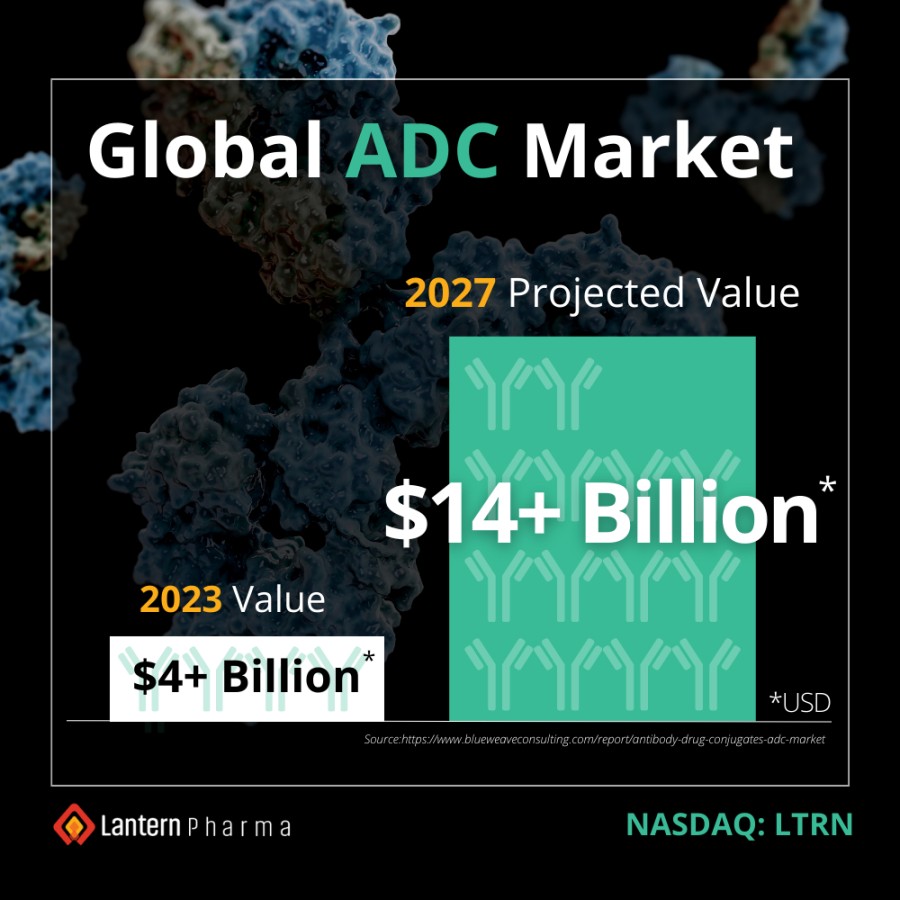
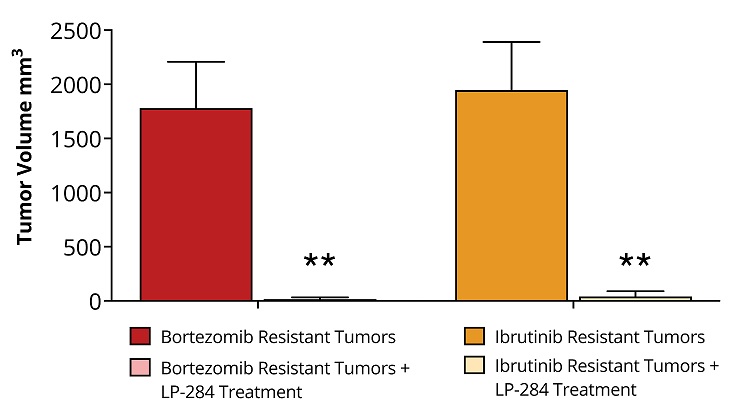
Multiple clinical trial sites across Taiwan are actively screening patients, following successful site initiation visits. Expansion into Taiwan is particularly significant as over 50% of lung cancer cases in Taiwan occur in never-smokers. DALLAS / Dec 09, 2024 / Business Wire / Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence (AI) company developing targeted cancer therapies using its proprietary RADR® AI... Read More