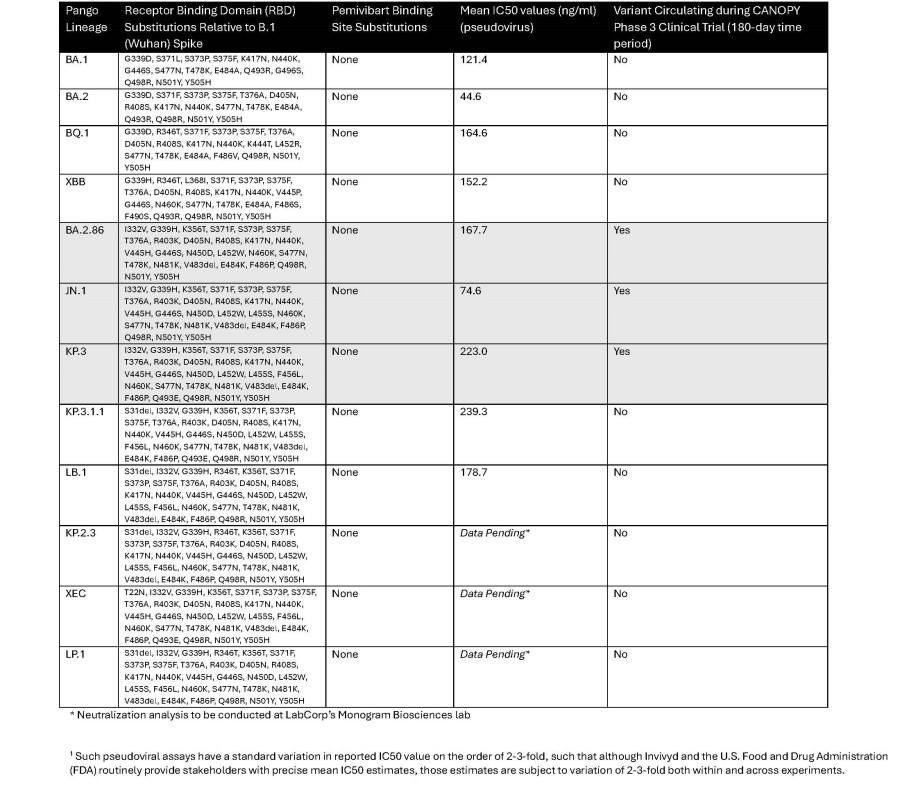
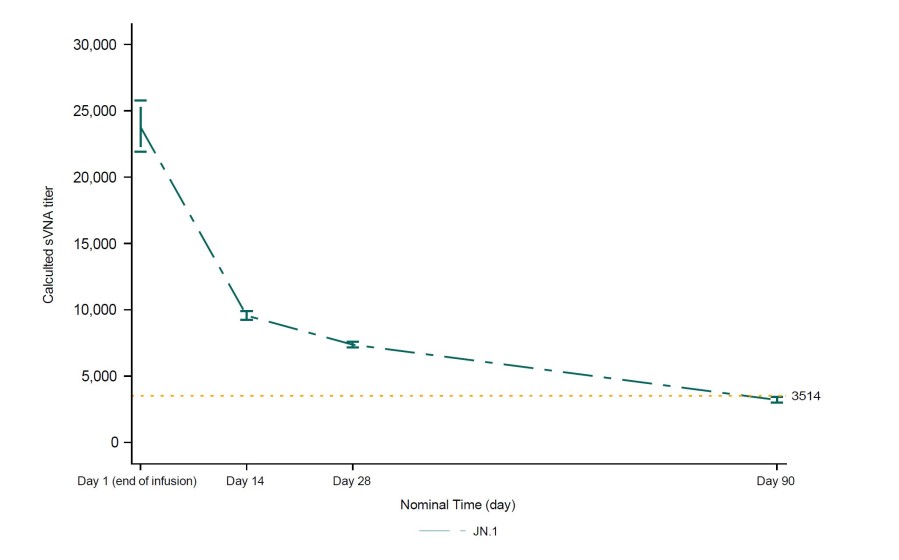
The New England Journal of Medicine (NEJM) Letter to the Editor outlines the novel, rapid immunobridging authorization pathway for PEMGARDA and provides an updated correlate of protection curve for monoclonal antibody protection from symptomatic COVID-19 The updated correlate of protection analysis published in the Letter to the Editor indicates the potential for strong protection from symptomatic COVID-19 at titer levels... Read More