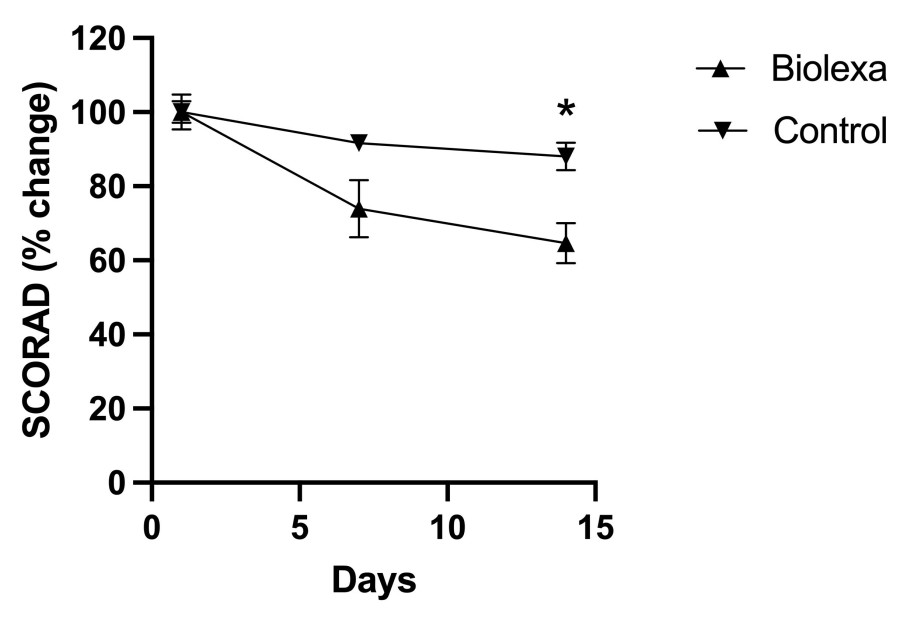
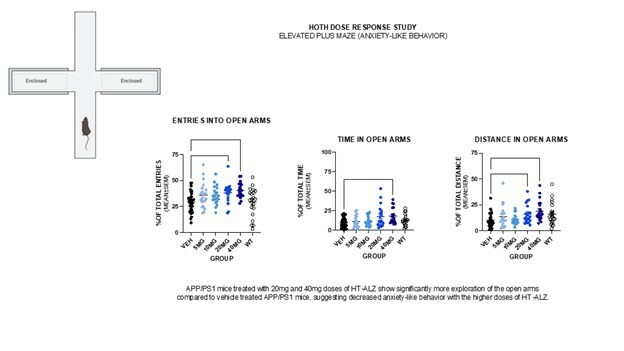
NEW YORK , Nov. 20, 2024 /PRNewswire/ -- Hoth Therapeutics, Inc. (NASDAQ: HOTH), a patient focused biopharmaceutical company, today announced that the Company's Board of Directors approved the purchase of up to $1 million in Bitcoin . "As Bitcoin continues to grow, gaining investor attention and acceptance as a major and primary asset class, we believe that Bitcoin will serve as a strong treasury reserve asset," said Robb... Read More