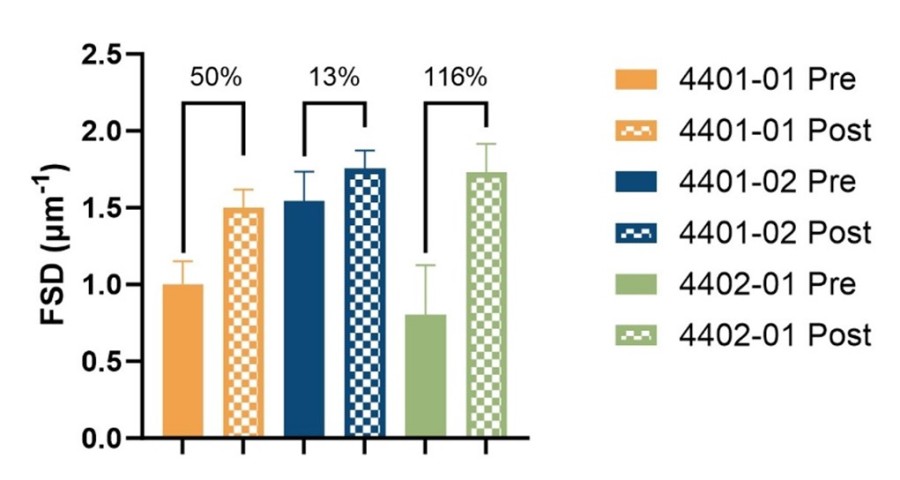
Kidney morphology improved in all three patients with protein re-expression consistent with disease regression in its Phase 2 open-label clinical trial of ELX-02 for the treatment of Alport Syndrome ELX-02 improved podocyte foot process effacement in all three treated patients by an average of 60% based on blinded biopsy analysis by NIPOKA GmbH Clinical data from Phase 2 study of ELX-02 for Alport Syndrome included in... Read More