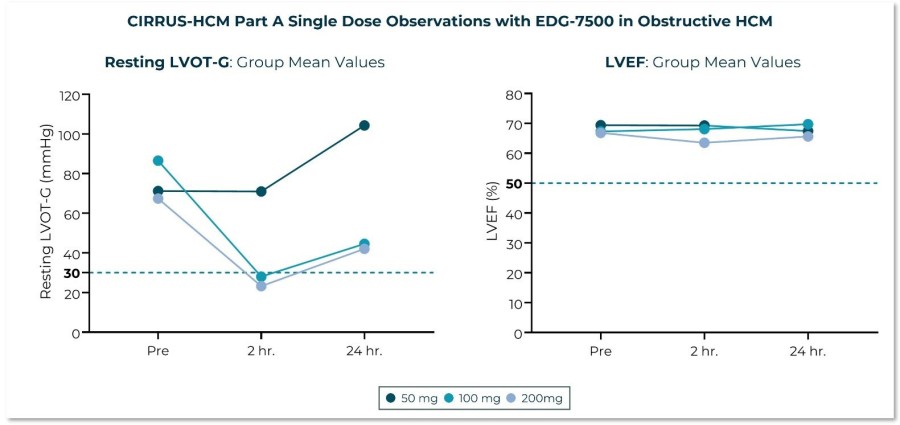
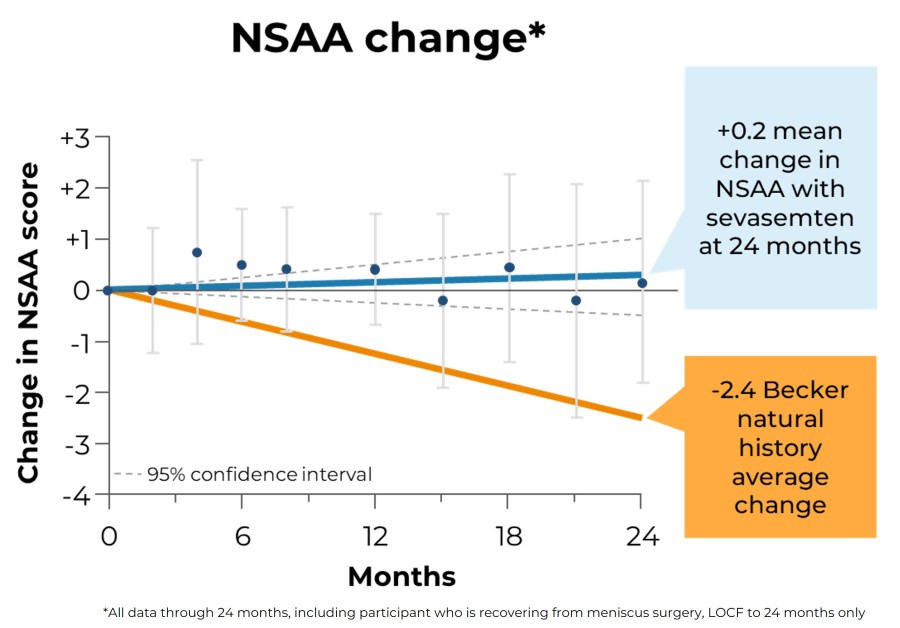
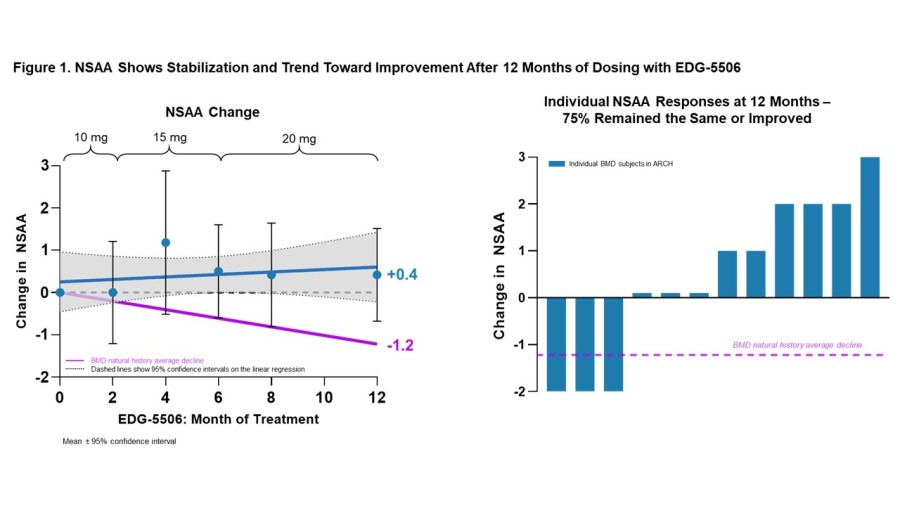
Trial met primary endpoint of reduction in circulating levels of creatine kinase (CK), a biomarker associated with skeletal muscle damage, in the largest Becker interventional trial to date On the key secondary endpoint, sevasemten-treated patients showed stabilization of North Star Ambulatory Assessment (NSAA) with a trend towards improvement at 12 months compared to placebo Sevasemten was well-tolerated and no new safety... Read More