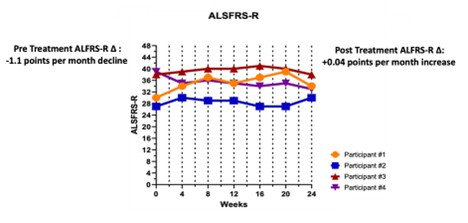
This open-label Phase 1 study measures safety, peripheral and central inflammation, effects on Treg cell populations, and FTD progression; Five of the 8 planned FTD subjects have been enrolled to date; Results of study will inform Coya’s randomized, double-blinded Phase 2 trial of COYA 302 in patients with FTD HOUSTON / Dec 18, 2024 / Business Wire / Coya Therapeutics, Inc. (NASDAQ: COYA) (“Coya” or the “Company”), a... Read More