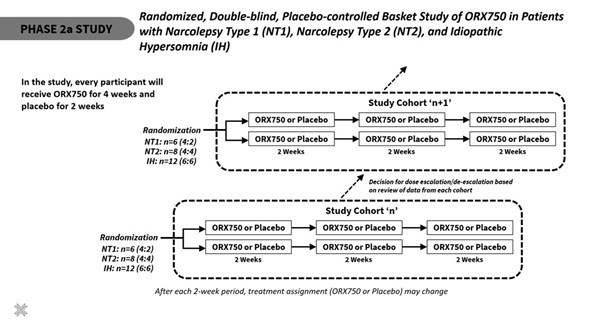
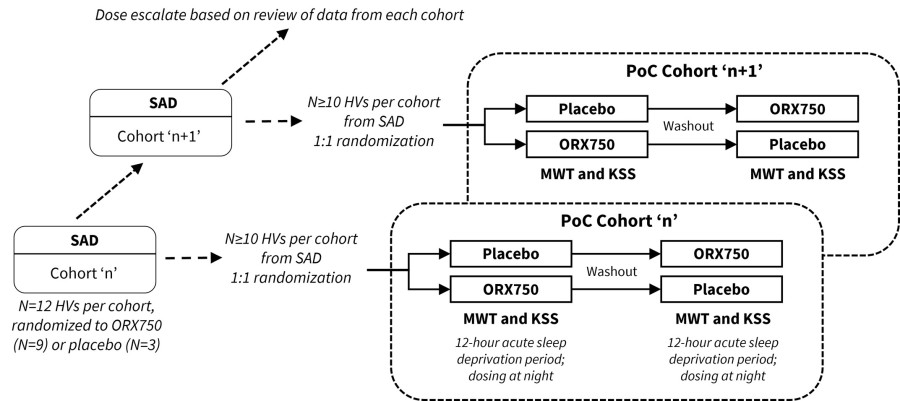
Announced additional interim data from ongoing Phase 1 clinical study of ORX750, a novel orexin receptor 2 (OX2R) agonist, in acutely sleep-deprived healthy volunteers that further support best-in-class potential of ORX750 in narcolepsy type 1 (NT1), narcolepsy type 2 (NT2), and idiopathic hypersomnia (IH); Presentation of... Read More