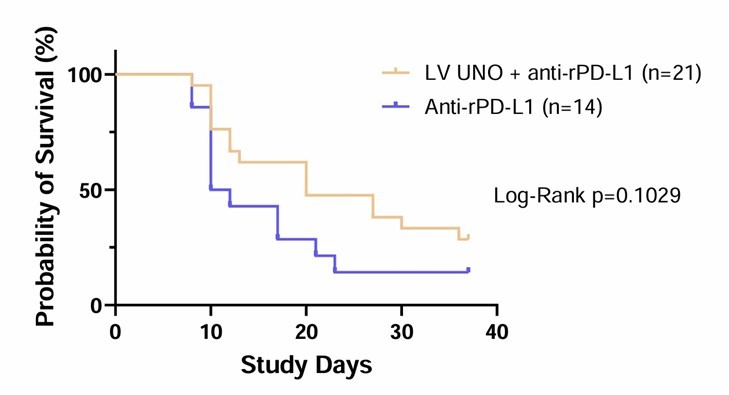
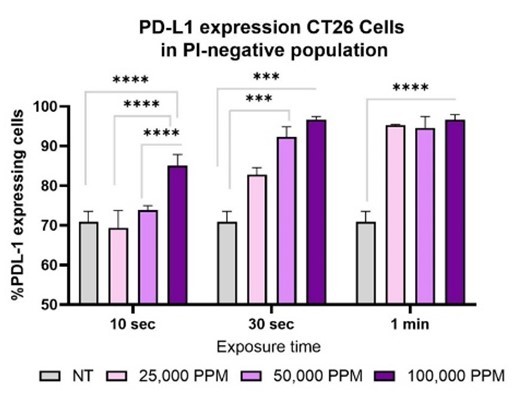
The Phase 1b study will evaluate LV UNO in unresectable cutaneous or subcutaneous histologically confirmed primary or metastatic solid tumor cancer patients that have progressed or have prolonged stable disease on single agent PD-1 inhibitors The primary objective of the Phase 1b study is to assess preliminary efficacy by objective response rate (ORR) and duration of response (DOR) per RECIST version 1.1 and secondarily... Read More