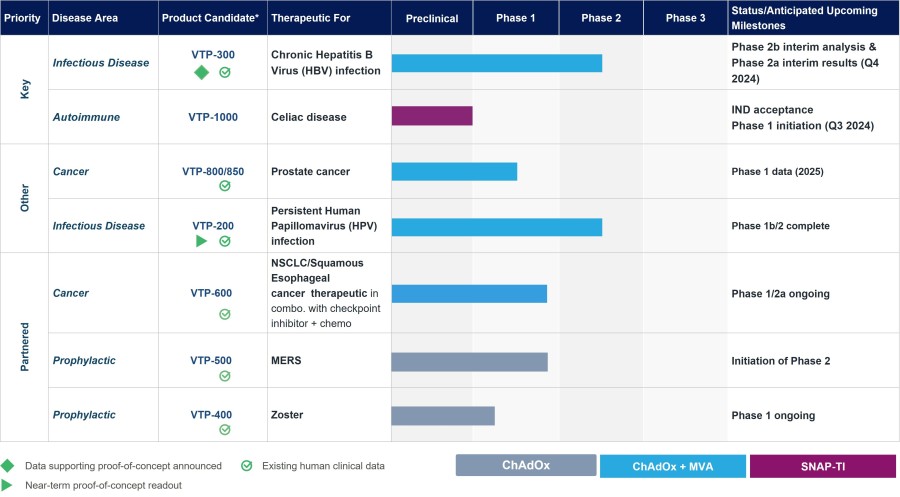
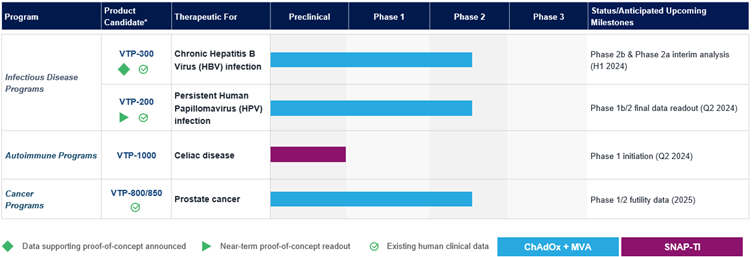
OXFORD, United Kingdom and GERMANTOWN, Md., Nov. 25, 2024 (GLOBE NEWSWIRE) -- Barinthus Biotherapeutics plc (NASDAQ: BRNS) (“Barinthus Bio”), today announced the promotion of Geoffrey Lynn, M.D., Ph.D. to Chief Scientific Officer (CSO), effective as of December 1, 2024. Dr. Lynn succeeds Nadège Pelletier, Ph.D. who decided to pursue alternative opportunities closer to home after having served as Barinthus Bio’s CSO since... Read More