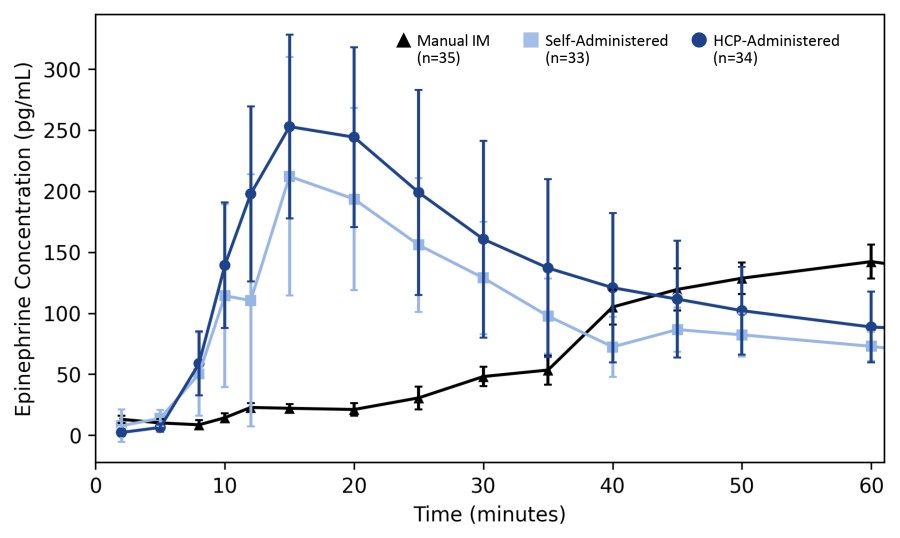
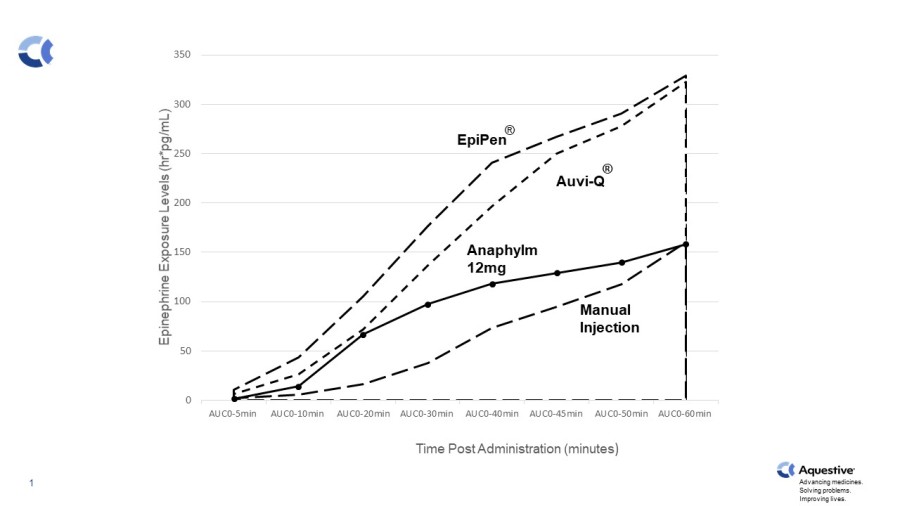
WARREN, N.J., Dec. 19, 2024 (GLOBE NEWSWIRE) -- Aquestive Therapeutics, Inc. (NASDAQ: AQST) (“Aquestive” or the “Company”), a pharmaceutical company advancing medicines to bring meaningful improvement to patients' lives through innovative science and delivery technologies, today announced the U.S. Food and Drug Administration (FDA) has granted seven years of orphan drug exclusivity (ODE) to Libervant® (diazepam) Buccal Film... Read More