DUBLIN, Jan. 8, 2024 /PRNewswire/ -- Medtronic plc (NYSE:MDT), a global leader in healthcare technology, today announced CE (Conformité Européenne) Mark approval for the MiniMed™ 780G system with Simplera Sync™, a disposable, all-in-one continuous glucose monitor (CGM) requiring no fingersticks or overtape. Simplera Sync™ features an improved user experience with a simple, two-step insertion process and is half the size of previous Medtronic sensors.*
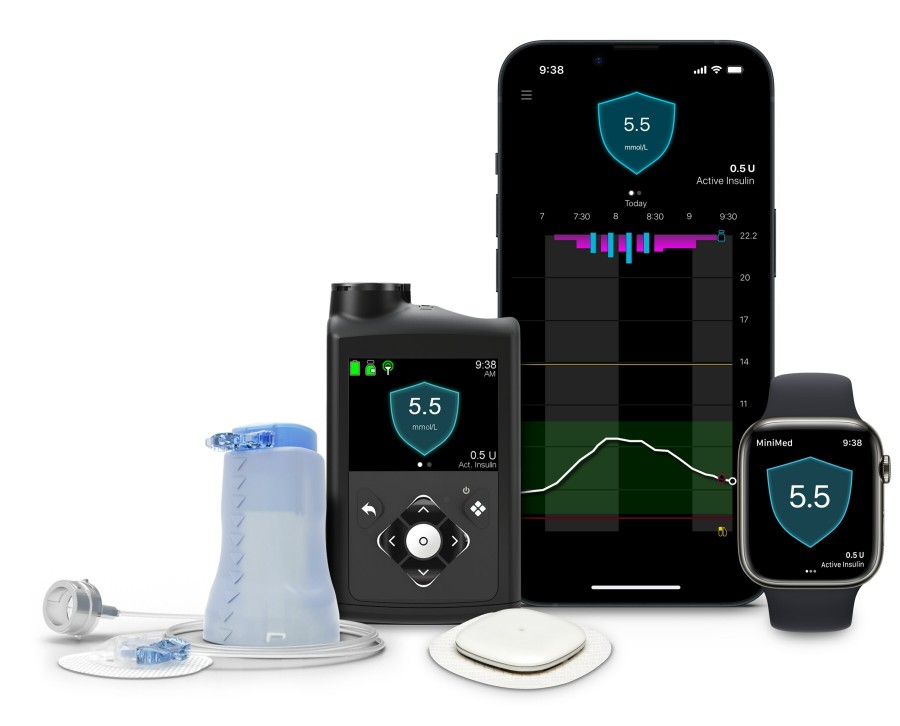
The MiniMed 780G system with Simplera Sync™ sensor will be available in Europe via limited release in spring 2024. Medtronic will begin the phased commercial launch in Europe in the summer of 2024. Today, the MiniMed™ 780G system can be used with the Guardian™ 4 sensor.
The MiniMed™ 780G system is Medtronic's most advanced insulin delivery system, automatically adjusting and correcting† glucose levels every 5 minutes.§ It's the world's only system with a Meal Detection™ feature‡ that is designed to reduce post-meal hyperglycemia when users occasionally forget to give themselves insulin or underestimate the number of carbs in their snacks or meal. The system, which is available with the world's only 7-day infusion set, also features one of the lowest glucose target settings (as low as 100 mg/dL) of any automated insulin delivery system. With this "treat to target" approach, the system more closely mirrors the glucose levels of someone not living with diabetes. With both Simplera Sync™ sensor and the Guardian™ 4 sensor, no fingersticks are required.**
"A challenging aspect of living with diabetes is counting carbohydrates and dosing the right amount of insulin before consuming snacks and meals. Many people underestimate their carbs, which can lead to high blood sugars (hyperglycemia). Prolonged hyperglycemia can lead to serious health problems impacting the eyes, major organs, and even cognitive function, which is particularly concerning in developing children," Robert Vigersky, M.D., Chief Medical Officer, Medtronic Diabetes, Professor of Medicine, Uniformed Services University of the Health Sciences "With its responsive algorithm, the MiniMed™ 780G system can help people living with diabetes even when they occasionally forget to bolus or undercount their carbs. The system takes on more of the work involved in diabetes management and helps alleviate mental burden."
"We're incredibly proud that the MiniMed™ 780G system continues to be the most widely used automated insulin delivery system in Europe since we launched it in 2020. Real-world data on over 100,000 users on the system across many geographies and cultures shows that when using recommended settings the system is delivering an average Time in Range of nearly 80%, raising the bar on what 'good' looks like2," said Que Dallara, EVP and President, Medtronic Diabetes. "With the introduction of Simplera Sync™ sensor, we're able to offer the proven benefits of our MiniMed™ 780G system with our newest and most comfortable sensor that can be applied in under 10 seconds."
The MiniMed™ 780G system with Simplera Sync™ sensor is indicated for ages 7+ and compatible with iOS and Android. Simplera Sync™ sensor is not approved by the FDA and is limited to investigational use in the U.S.
Simplera Sync™ sensor is designed to leverage Medtronic's advanced AID algorithm as part of its MiniMed™ 780G system while having a similar look and feel as the Simplera™ CGM. The Simplera™ CGM for integrated use with the InPen™ smart insulin pen received CE Mark in September 2023.
About the Diabetes Business at Medtronic (www.medtronicdiabetes.com)
Medtronic Diabetes is on a mission to alleviate the burden of diabetes by empowering individuals to live life on their terms, with the most advanced diabetes technology and always-on support when and how they need it. We've pioneered first-of-its-kind innovations for over 40 years and are committed to designing the future of diabetes management through next-generation sensors (CGM), intelligent dosing systems, and the power of data science and AI while always putting the customer experience at the forefront.
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Dublin, Ireland, is the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 90,000+ passionate people across more than 150 countries. Our technologies and therapies treat 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary. For more information on Medtronic (NYSE:MDT), visit www.Medtronic.com and follow @Medtronic on Twitter and LinkedIn.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
1. Data on file, CIP330 Evaluation of Updated Continuous Glucose Monitoring (CGM) Form Factor in Adults, Adolescents and Pediatrics.
2. Choudhary, Arrieta, van den Heuvel, Castaneda, Smaniotto, Cohen. Celebrating the data from 100,000 real-world users of the MiniMed 780G system in Europe, Middle East and Africa collected over 3 years: from data to clinical evidence. DTT Epub ahead of print. Doi: 10.1089/dia.2023.0433
*Participants in a clinical trial reported the sensor was smaller and lighter than other sensors they have used. Data on file: CIP330, N=243, ages 2-80.
†Refers to auto correct, which provides bolus assistance. Can deliver all correction doses automatically without user interaction, feature can be turned on and off.
‡Taking a bolus 15 – 20 minutes before a meal helps to keep blood sugar levels under control after eating.
§ Refers to SmartGuard™ feature. Individual results may vary.
**A blood glucose (BG) reading is needed when entering SmartGuard™ feature. If glucose alerts and CGM readings do not match your symptoms, use a BG meter to make diabetes treatment decisions. Refer to System User Guide – SmartGuard™ feature. Some user interaction required.
Media Kit: https://news.medtronic.com/MiniMed-TM-780G-System-with-Simplera-Sync-TM
Contacts: | |
Ashley Patterson | Ryan Weispfenning |
Public Relations | Investor Relations |
+1-818-576-3025 | +1-763-505-4626 |

| Last Trade: | US$81.03 |
| Daily Change: | 1.04 1.30 |
| Daily Volume: | 8,057,280 |
| Market Cap: | US$103.720B |
November 20, 2024 November 19, 2024 November 01, 2024 October 28, 2024 October 24, 2024 | |

Immix Biopharma is a clinical-stage biopharmaceutical company pioneering a novel class of CAR-T cell therapies and Tissue-Specific Therapeutics targeting oncology and immuno-dysregulated diseases with >75 patients treated to-date. Our lead cell therapy asset is NXC-201...
CLICK TO LEARN MORE
Cue Biopharma is developing the first-ever class of therapeutics for the treatment of cancer that mimic the natural signals, or “Cues”, of the immune system. This novel class of injectable biologics selectively engages and modulates tumor-specific T cells directly within the patient’s body to transform...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB