DUBLIN, Jan. 8, 2024 /PRNewswire/ -- Medtronic plc (NYSE:MDT), a global leader in healthcare technology, today announced the U.S. Food and Drug Administration (FDA) approval of its Percept™ RC Deep Brain Stimulation (DBS) system. The rechargeable neurostimulator is the latest innovation in the Medtronic Percept™ family, which includes the Percept™ PC neurostimulator, BrainSense™ technology†, and SenSight™ directional leads. The Percept™ family is the only sensing-enabled DBS system on the market, allowing the physician to personalize treatment for patients with movement disorders such as Parkinson's disease, essential tremor, and dystonia* as well as epilepsy. Over 11 million people in the U.S. are living with movement disorders1-2 and approximately 3.4 million with epilepsy3.
"Our DBS therapy with exclusive BrainSense™ technology can help control debilitating tremors for people living with Parkinson's, providing patients with the ability to physically engage in everyday moments – something many of us unintentionally take for granted," said Amaza Reitmeier, vice president and general manager, Brain Modulation within the Neuromodulation business, which is part of the Neuroscience Portfolio at Medtronic. "We are transforming brain modulation through sensing-enabled DBS and will continue to drive therapy innovation with the goal of many more peoples' lives improved with Medtronic DBS therapy."
DBS uses a surgically implanted medical device, similar to a cardiac pacemaker. Medtronic Percept™ neurostimulators transmit electrical signals via slender wires to specific brain targets affected by debilitating neurological disorders like Parkinson's disease.
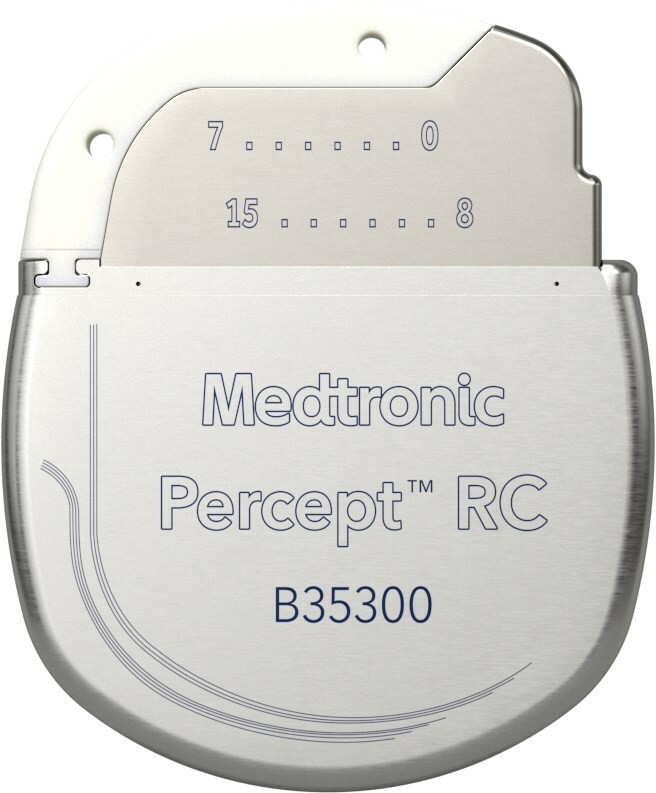
Percept™ RC is the smallest and thinnest dual channel‡ neurostimulator available for DBS. It is equipped with BrainSense™ technology that captures and records brain signals to provide insights that enable a healthcare provider to adapt and personalize therapy to a patient's evolving needs. Unlike other rechargeable devices, the Percept™ RC battery offers at least 15 years of service life with consistent and fast recharge performance. Medtronic patented battery technology has less battery fade than other rechargeable devices for a more reliable, long-lasting battery.§ Patients can experience rapid recharging from 10% to 90% full charge in less than an hour.Ω
"While more data are needed, the sensing capability of this unique deep brain stimulation system allows me the potential to tune stimulation delivery to brain activity, which may be a way to personalize this therapy for Parkinson's disease in the future," said Casey H. Halpern, M.D., Neurosurgeon and deep brain stimulation expert.
Nearly 70 percent of all DBS-eligible patients are estimated to require an MRI as part of their essential care4. Medtronic was the first in the United States to offer full-body MR Conditional DBS systems for patients to have safe scans anywhere on the body under specific conditions††.
Percept™ offers greater freedom and scan access for patients with 3T scans and best-in-class 1.5T MRI scan labeling5-7. Medtronic has the only DBS system that allows patients to have stimulation on in bipolar mode during an MRI5-7.†† In addition, the Percept™ neurostimulators are engineered to allow for future software updates designed for the Percept™ platform without a neurostimulator device exchange.
†† Under specific conditions. Refer to product labeling for full list of conditions: https://manuals.medtronic.com/manuals/mri/region
"This new rechargeable neurostimulator technology provides me with insights into my patients' symptoms and can capture data even when they're outside of the clinic," said Eleni Okeanis Vaou, M.D., FAAN, associate professor of neurology. "Now I have a rechargeable DBS therapy option with sensing technology allowing me to track a patient's response to DBS and medications. I use this data to inform how to personalize and optimize patient therapy."
Dr. Vaou is a deep brain stimulation expert with the University of Texas Health Science Center at San Antonio (UT Health San Antonio) and is not affiliated with Medtronic. She and the institution do not endorse products or services.
Since 1987, Medtronic has served over 180 thousand patients in more than 70 countries with its life-changing DBS therapy8. Percept™ RC is available immediately throughout the U.S., as well as via CE Mark approval in Europe and availability in Japan.
For further information on the Percept™ neurostimulators with exclusive BrainSense™ technology, visit our website here.
About Medtronic
Bold thinking. Bolder actions. We are Medtronic. Medtronic plc, headquartered in Dublin, Ireland, is the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 95,000+ passionate people across 150 countries. Our technologies and therapies treat 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary. For more information on Medtronic (NYSE:MDT), visit www.Medtronic.com and follow @Medtronic LinkedIn.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
Medtronic DBS therapy is approved for five indications: Parkinson's disease, essential tremor, dystonia*, obsessive-compulsive disorder* (OCD), and epilepsy. Indications vary by product. Refer to product labeling for details.
*Humanitarian device: The effectiveness of these devices for the treatment of dystonia and obsessive-compulsive disorder has not been demonstrated.
† The sensing feature of the Percept™ PC and Percept™ RC system is intended for use in patients receiving DBS where chronically recorded bioelectric data may provide useful, objective information regarding patient clinical status. The majority of patients with Parkinson's disease have an identifiable signal. Signal may not be present or measurable in patients treated for essential tremor, dystonia*, epilepsy or obsessive-compulsive disorder*. *Humanitarian device: The effectiveness of these devices for the treatment of dystonia or obsessive-compulsive disorder has not been demonstrated.
‡ As compared to Boston Scientific Vercise Genus™* R16 and Vercise Genus™* P16. MP92328632-05 REV-A. As compared to St Jude Medical Infinity™* 5/7 IPG. ARTEN600150429 - B.
§The Boston Scientific Vercise Genus™* R16 has a variable 5–15 years of service life, depending on the stimulation settings and conditions (Vercise™* Deep Brain Stimulation Systems Information for Prescribers MP92366224-01 Rev G, accessed August 22, 2023).
Ω For implant depths of up to 2.0 cm under normal conditions.
References
1. Statistics on Parkinson's. Parkinson's Disease Foundation Web site. https://www.parkinson.org/understanding-parkinsons/statistics. Accessed 11/1/23
2. Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25(5):534-541.
3. https://www.epilepsy.com Accessed 11/1/23
4. Falowski S, Safriel Y, Ryan MP, Hargens L. The rate of magnetic resonance imaging in patients with deep brain stimulation. Stereotact Funct Neurosurg. 2016; 94(3):147-153
5. ImageReady™ MRI Guidelines for Boston Scientific Deep Brain Stimulation Systems — 92195369-01, accessed on 10/18/2023
6. MRI Procedure Information for Abbott Medical™* MR Conditional Deep Brain Stimulation Systems —ARTEN600090482 A. Accessed on October 18, 2023.
7. MRI guidelines for Medtronic deep brain stimulation systems 37601 37602 37603 37612 B35200 B35300 — M929535A_a_092 https://manuals.medtronic.com/manuals/mri/region
8. Medtronic data on file.
UC202407968 EN
Contacts: | |
Naomi Rodiles | Ryan Weispfenning |
Public Relations | Investor Relations |
+1-612-427-5521 | +1-763-505-4626 |

| Last Trade: | US$80.03 |
| Daily Change: | -1.34 -1.65 |
| Daily Volume: | 6,335,448 |
| Market Cap: | US$102.440B |
November 20, 2024 November 19, 2024 November 01, 2024 October 28, 2024 October 24, 2024 | |

Immix Biopharma is a clinical-stage biopharmaceutical company pioneering a novel class of CAR-T cell therapies and Tissue-Specific Therapeutics targeting oncology and immuno-dysregulated diseases with >75 patients treated to-date. Our lead cell therapy asset is NXC-201...
CLICK TO LEARN MORE
Chimerix is on a mission to develop medicines that meaningfully improve and extend the lives of patients facing deadly diseases. The company is devoted to filling gaps in the treatment paradigm. Chimerix’s most advanced clinical-stage program is in development for H3 K27M-mutant glioma....
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB