Encinitas, California--(Newsfile Corp. - March 25, 2024) - Kiora Pharmaceuticals, Inc. (NASDAQ: KPRX) ("Kiora" or the "Company") today announced its 2023 financial results and provided an update on its retinal disease development pipeline. The Company's major initiatives planned for 2024 are to initiate Phase 2 clinical development of KIO-301, a small molecule photoswitch, for the treatment of inherited retinal diseases, starting with retinitis pigmentosa (RP); and further development of KIO-104, an intravitreal, anti-inflammatory for treatment of non-infectious uveitis. The $16 million upfront payment from Kiora's development and commercialization partnership with Théa Open Innovation (TOI) for KIO-301 and its $15 million private placement are expected to be sufficient to fund the Company through 2026, excluding any potential partnership milestones or warrant exercises.
"Our greatest priorities this year are to advance KIO-301 and KIO-104 to assess their further potential to benefit patients," said Brian M. Strem, Ph.D., chief executive officer of Kiora. "Both products are clinically validated drug candidates based on proprietary and innovative small molecules targeting rare retinal diseases with large unmet needs.
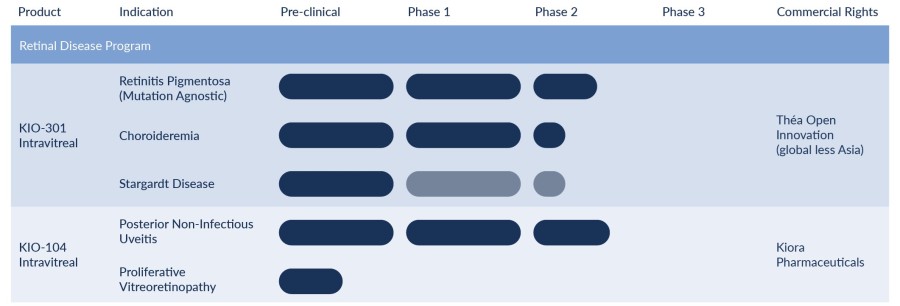 Kiore’s pipeline of drug candidates against rare retinal diseases.
Kiore’s pipeline of drug candidates against rare retinal diseases.
"KIO-301 could potentially become the first vision restoring option for patients with inherited retinal degenerative diseases like RP. Ongoing development will be collaboratively guided and fully funded by our partner, TOI. Should KIO-301 gain marketing authorization, it would provide Kiora with meaningful commercial milestones and royalties up to the low 20s (%) with a partner who has a proven track-record of leadership in the eye space. Working with TOI, we are finalizing details of the randomized, double-masked, controlled, dose-ascending Phase 2 trial in RP and look forward to providing more specifics on expected enrollment and results timelines.
"The balance sheet provides us the financial ability to fund further development of KIO-104 for the treatment of posterior non-infectious uveitis. Because non-infectious uveitis can be sight threatening, there is a strong market need for new, steroid sparing therapeutic approaches. The active compound in KIO-104 is a highly potent, disease modifying anti-inflammatory agent belonging to a class of drugs helping hundreds of thousands of patients with systemic autoimmune diseases including multiple sclerosis and rheumatoid arthritis. By delivering KIO-104 directly to the eye, we believe we can reduce the negative effects of retinal inflammation without the associated potential side effects of systemic anti-inflammatory drugs or chronic steroid exposure. Beyond non-infectious uveitis, the mechanism of action of KIO-104 could apply to other retinal conditions, such as proliferative vitreoretinopathy (PVR), a complication following retinal detachment repair, where nonclinical work is ongoing."
"Orphan indications involve efficient, cost-effective paths to market," added Melissa Tosca, EVP Finance. "For 2024, we expect to increase our R&D spend, with expenses for KIO-301 offset by quarterly reimbursement from TOI. Further, we anticipate G&A expenses to remain relatively flat for the year. We believe this balances our desire to achieve meaningful development while maintaining a strong cash position expected to fund operations through 2026."
 Kiora has strengthened its fundamentals by advancing its pipeline, entering a strategic partnership, and strengthening its balance sheet.
Kiora has strengthened its fundamentals by advancing its pipeline, entering a strategic partnership, and strengthening its balance sheet.
Achieved and Upcoming Milestones:
Notable milestones that Kiora achieved in 2023 and year-to-date 2024 include the following:
KIO-301
KIO-100 Family (KIO-104, KIO-101)
Kiora anticipates achieving the following clinical and regulatory milestones:
KIO-301
KIO-104
Financial Results
Kiora ended the year with $2.5 million in cash and cash equivalents and $2.0 million in tax receivables. In February of 2024, Kiora received an additional $16 million for an upfront payment from TOI and raised $13.8 million in net proceeds from a private placement offering. The Company's cash and cash equivalents as of March 24, 2024, exceeds $30 million and the Company believes this will fund operations through 2026.
In 2023, research and development expenses were $4.0 million, net of $1.7 million in offsetting tax credits, compared to $3.5 million, net of $1.5 million in offsetting tax credits, for 2022. Research and development expenses for the fourth quarter of 2023 were $1.1 million, net of $0.5 million in offsetting tax credits, compared to $0.8 million, net of $0.4 million in offsetting tax credits for the fourth quarter of 2022. The increase in R&D in 2023 was primarily due to greater investment in clinical trial-related activities for KIO-301 and personnel-related expenses for the R&D team. Kiora anticipates R&D expenses will increase as it begins planning and enrollment of patients in Phase 2 clinical trials, with trial-related expenses for KIO-301 offset by reimbursement from TOI.
General and administrative expenses for 2023 were $4.7 million compared to $8.3 million for 2022. General and administrative expenses for the fourth quarter of 2023 were $0.9 million, compared to $2.8 million in the fourth quarter of 2022. The reduction in general and administrative expenses for the full year and fourth quarter of 2023 were primarily related to lower professional service fees, driven by reduced external accounting and auditing services. Kiora expects that general and administrative expenses will remain relatively consistent for the near future.
Net loss was $12.5 million for 2023 compared to $13.6 million for 2022. Net loss was $2.3 million for the fourth quarter of 2023 compared to $2.5 million for the fourth quarter of 2022.
About Kiora Pharmaceuticals
Kiora Pharmaceuticals is a clinical-stage biotechnology company developing and commercializing products for the treatment of orphan retinal diseases. KIO-301 is being developed for the treatment of retinitis pigmentosa, choroideremia, and Stargardt disease. It is a molecular photoswitch that has the potential to restore vision in patients with inherited and/or age-related retinal degeneration. KIO-104 is being developed for the treatment of posterior non-infectious uveitis. It is a next-generation, non-steroidal, immuno-modulatory, and small-molecule inhibitor of dihydroorotate dehydrogenase. In addition to news releases and SEC filings, we expect to post information on our website, www.kiorapharma.com, and social media accounts that could be relevant to investors. We encourage investors to follow us on Twitter and LinkedIn as well as to visit our website and/or subscribe to email alerts.
Forward-Looking Statements
Some of the statements in this press release are "forward-looking" and are made pursuant to the safe harbor provision of the Private Securities Litigation Reform Act of 1995. These "forward-looking" statements include statements relating to, among other things, Kiora's ability to execute on development and commercialization efforts and other regulatory or marketing approval efforts pertaining to Kiora's development-stage products, including KIO-104, KIO-301, KIO-201 and KIO-101, as well as the success thereof, with such approvals or success may not be obtained or achieved on a timely basis or at all, the sufficiency of existing cash on hand to fund operations for specific periods, the ability to timely complete planned initiatives for 2024, including phase 2 clinical development of KIO-301 and KIO-104, the potential for KIO-301 to be the first treatment options for patients with inherited degenerative diseases like RP, Kiora's plans to further fund development of KIO-104, the potential for KIO-104 to reduce inflammation, the timing of topline results from a Phase 2b trial of KIO-104, the potential for KIO-104 to apply to other retinal inflammatory diseases, and expected trends for research and development and general and administrative spending in 2024. These statements involve risks and uncertainties that may cause results to differ materially from the statements set forth in this press release, including, among other things, the ability to satisfy the closing conditions related to the offering ,the ability to conduct clinical trials on a timely basis, market and other conditions and certain risk factors described under the heading "Risk Factors" contained in Kiora's Annual Report on Form 10-K filed with the SEC on March 25, 2024 or described in Kiora's other public filings. Kiora's results may also be affected by factors of which Kiora is not currently aware. The forward-looking statements in this press release speak only as of the date of this press release. Kiora expressly disclaims any obligation or undertaking to release publicly any updates or revisions to such statements to reflect any change in its expectations with regard thereto or any changes in the events, conditions, or circumstances on which any such statement is based, except as required by law.
Contacts:
Investors
This email address is being protected from spambots. You need JavaScript enabled to view it.
Media
This email address is being protected from spambots. You need JavaScript enabled to view it.
| Last Trade: | US$3.20 |
| Daily Change: | -0.07 -2.14 |
| Daily Volume: | 16,419 |
| Market Cap: | US$9.600M |
November 08, 2024 August 09, 2024 July 01, 2024 | |

Astria Therapeutics is a biopharmaceutical company, and our mission is to bring life-changing therapies to patients and families affected by rare and niche allergic and immunological diseases. Our lead program, STAR-0215, is a monoclonal antibody inhibitor of plasma kallikrein in clinical development...
CLICK TO LEARN MORE
Amneal Pharmaceuticals is a fully-integrated essential medicines company. We make healthy possible through the development, manufacturing, and distribution of generic and specialty pharmaceuticals. The Company has a diverse portfolio of over 250 products in its Generics segment and is expanding across...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB