TORONTO and HAIFA, Israel, Aug. 09, 2024 (GLOBE NEWSWIRE) -- NurExone Biologic Inc. (TSXV: NRX) (OTCQB: NRXBF) (Germany: J90) (the “Company” or “NurExone”) is pleased to announce new data for its ExoPTEN nanodrug, marking a significant step towards commercial-grade manufacturing. Building on the announcement of a new Good Manufacturing Practice (“GMP”) compliant Contract Research Organization (“CRO”) partner, this study assessed the performance of ExoPTEN loaded with small interfering RNA (“siRNA”) produced by the new manufacturer (the “GMP Partner”).
This study focused on the capability of ExoPTEN to biologically target sites of inflammation and injury as evidenced by a high concentration of the drug in damaged tissue. ExoPTEN, loaded with siRNA either from the GMP Partner or from a research grade CRO, was minimally-invasively administered to rats after spinal cord compression injury. The treated rats were compared to each other and to an untreated control group. The homing capacity of ExoPTEN was assessed by evaluating biodistribution of the ExoPTEN three days post-injury and injection.
As shown in Figure 1 below, ExoPTEN loaded with siRNA from both sources (A and C) demonstrated exceptional homing capacity to the injured spinal cord, targeting the site of inflammation with precision. This resulted in a high concentration of the drug in damaged tissue, further validating the quality of the siRNA produced by the Company’s GMP Partner and the use of NurExone’s exosomes as a drug delivery system.
“We are excited by the successful results of the highly complex transfer to a commercial manufacturer,” commented Dr. Noa Avni, Director of Research and Development. “These positive results reinforce our confidence in our ability to produce and scale up our siRNA to meet the quality and regulatory standards needed for commercial manufacturing. It also shows the scalability and reliability of our therapies as we advance towards clinical trials,” Dr. Avni added.
Dr. Lior Shaltiel, CEO of NurExone, also noted, “The ability of our loaded exosomes to precisely target sites of inflammation underscores their potential as an ideal and natural choice for drug delivery. We continue to be enthused by the progress we are observing.”
Figure 1: Homing ability of ExoPTEN using exosomes loaded with siRNA manufactured from a GMP-Compliant Manufacturer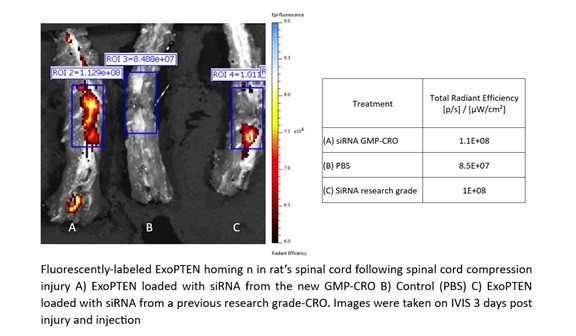
About NurExone Biologic Inc.
NurExone Biologic Inc. is a TSX Venture Exchange (“TSXV”) listed pharmaceutical company that is developing a platform for biologically guided exosome-based therapies to be delivered, non-invasively, to patients who have suffered Central Nervous System injuries. The Company’s first product, ExoPTEN for acute spinal cord injury, was proven to recover motor function in 75% of laboratory rats when administered intranasally. ExoPTEN has been granted Orphan Drug Designation by the FDA. The NurExone platform technology is expected to offer novel solutions to drug companies interested in noninvasive targeted drug delivery for other indications.
For additional information, please visit www.nurexone.com or follow NurExone on LinkedIn, Twitter, Facebook, or YouTube.
For more information, please contact:
Dr. Lior Shaltiel
Chief Executive Officer and Director
Phone: +972-52-4803034
Email: This email address is being protected from spambots. You need JavaScript enabled to view it.
Thesis Capital Inc.
Investment Relation - Canada
Phone: +1 905-347-5569
Email: This email address is being protected from spambots. You need JavaScript enabled to view it.
Dr. Eva Reuter
Investment Relation - Germany
Phone: +49-69-1532-5857
Email: This email address is being protected from spambots. You need JavaScript enabled to view it.
FORWARD-LOOKING STATEMENTS
This press release contains certain “forward-looking statements” that reflect the Company’s current expectations and projections about its future results. Wherever possible, words such as “may”, “will”, “should”, “could”, “expect”, “plan”, “intend”, “anticipate”, “believe”, “estimate”, “predict” or “potential” or the negative or other variations of these words, or similar words or phrases, have been used to identify these forward-looking statements. Forward-looking statements in this press release include, but are not limited to, statements relating to the success of the partnership with the GMP Partner; the Company making progress in its new study focused on the capability of the ExoPTEN exosomes loaded with siRNA; the results of the study and implications of the study; the Company’s ability to produce and scale up the siRNA; the exosomes becoming an ideal and natural choice for drug delivery; the GMP Partner partnership helping NurExone continue its development of its ExoPTEN nanodrug, which it hopes to use to treat central nervous system injuries; and the homing capacity of the ExoPTEN exosomes loaded with siRNA.
These statements reflect management’s current beliefs and are based on information currently available to management as at the date hereof. In developing the forward-looking statements in this press release, we have applied several material assumptions, including the general business and economic conditions of the industries and countries in which we operate; the general market conditions; the ability to secure additional funding; the partnership with the GMP manufacturer having the intended impact on the Company and its business; the patents safeguarding NurExone’s technology; the Company’s drug products having its intended benefits and effects; the Company making progress through new partnerships and technologies to move towards commercialization of their products; the Company’s intellectual property and technology being novel and inventive; the intellectual property having the intended impact on the Company and its business; the ExoPTEN exosomes loaded with siRNA having its intended benefits; the Company producing and scaling up the siRNA; the exosomes becoming an ideal and natural choice for drug delivery; the GMP Partner partnership helping NurExone continue its development of its ExoPTEN nanodrug, which it hopes to use to treat central nervous system injuries; and the NurExone platform technology offering novel solutions to drug companies.
Forward-looking statements involve significant risk, uncertainties and assumptions. Many factors could cause actual results, performance or achievements to differ materially from the results discussed or implied in the forward-looking statements. These risks and uncertainties include, but are not limited to risks related to the Company’s early stage of development; lack of revenues to date; government regulation; market acceptance for its products; rapid technological change; dependence on key personnel; protection of the Company’s intellectual property; dependence on the Company’s strategic partners; the fact that preclinical drug development is uncertain, and the drug product candidates of the Company may never advance to clinical trials; the fact that results of preclinical studies and early-stage clinical trials may not be predictive of the results of later stage clinical trials; the uncertain outcome, cost, and timing of product development activities, preclinical studies and clinical trials of the Company; the uncertain clinical development process, including the risk that clinical trials may not have an effective design or generate positive results; the potential inability to obtain or maintain regulatory approval of the drug product candidates of the Company; the introduction of competing drugs that are safer, more effective or less expensive than, or otherwise superior to, the drug product candidates of the Company; the initiation, conduct, and completion of preclinical studies and clinical trials may be delayed, adversely affected or impacted by unforeseen issues; the potential inability to obtain adequate financing; the potential inability to obtain or maintain intellectual property protection for the drug product candidates of the Company; the NurExone platform technology being unable to offer novel solutions to drug companies; risks that the Company’s intellectual property and technology won’t have the intended impact on the Company and/or its business; the Company’s inability to realize upon partnership with the new GMP Partner; the Company’s inability to produce and scale up the siRNA; risk that the exosomes will not become an ideal and/or natural choice for drug delivery; and the risks discussed under the heading “Risk Factors” on pages 29 to 36 of the Company’s Annual Information Form dated March 30, 2023, a copy of which is available under the Company’s SEDAR+ profile at www.sedarplus.ca. These factors should be considered carefully, and readers should not place undue reliance on the forward-looking statements. Although the forward-looking statements contained in this press release are based upon what management believes to be reasonable assumptions, the Company cannot assure readers that actual results will be consistent with these forward-looking statements. These forward-looking statements are made as of the date of this press release, and the Company assumes no obligation to update or revise them to reflect new events or circumstances, except as required by law.
Neither TSXV nor its Regulation Services Provider (as that term is defined in the policies of the TSXV) accepts responsibility for the adequacy or accuracy of this release.

| Last Trade: | C$0.62 |
| Daily Volume: | 0 |
| Market Cap: | C$43.950M |
December 06, 2024 November 27, 2024 November 13, 2024 November 01, 2024 September 26, 2024 | |

Recursion Pharmaceuticals is a clinical stage TechBio company leading the space by decoding biology to industrialize drug discovery. Enabling its mission is the Recursion OS, a platform built across diverse technologies that continuously expands one of the world’s largest....
CLICK TO LEARN MORE
C4 Therapeutics is pioneering a new class of small-molecule drugs that selectively destroy disease-causing proteins via degradation using the innate machinery of the cell. This targeted protein degradation approach offers advantages over traditional drugs, including the potential to treat a wider range of diseases...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB