This news release constitutes a “designated news release” for the purposes of Cybin’s prospectus supplements each dated August 23, 2023, to its short form base shelf prospectus dated August 17, 2023, as amended December 22, 2023 and April 8, 2024.
TORONTO / Nov 13, 2024 / Business Wire / Cybin Inc. (NYSE American:CYBN) (Cboe CA:CYBN) (“Cybin” or the “Company”), a clinical-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options, today announced the initiation of PARADIGMTM, its Phase 3 pivotal program evaluating the efficacy and safety of CYB003 for the adjunctive treatment of Major Depressive Disorder (“MDD”). The program name, PARADIGM, represents the Company's belief that CYB003 could have the potential for a paradigm shift in the treatment of depression. The Company also today reported unaudited financial results for its second quarter ended September 30, 2024.
“Just three years after filing an Investigational New Drug application for CYB003, the initiation of our Phase 3 program is a truly significant and gratifying milestone,” said Doug Drysdale, Chief Executive Officer of Cybin. “Following a highly collaborative and thorough design and review process with the U.S. Food and Drug Administration, we believe that PARADIGM incorporates appropriate protocols that proactively address some of the challenges encountered by peers developing molecules with similar mechanisms of action by (i) recruiting from the larger MDD population; (ii) administering CYB003 as an adjunctive treatment and not requiring patients to titrate off their existing antidepressants; and (iii) utilizing a 12-week blinded period to maximize the number of patients that remain in the study through the blinded stage. Our innovative approach represents a potential ‘paradigm’ shift, moving away from the daily treatment of depression symptoms and toward an intermittent, more durable treatment like CYB003 that could potentially change the course of the disease. Our clinical team has accomplished an extraordinary amount in a short time, and we are eager to continue investigating CYB003’s potential to provide long-lasting relief from depressive symptoms and disrupt the standard of care in MDD,” concluded Drysdale.
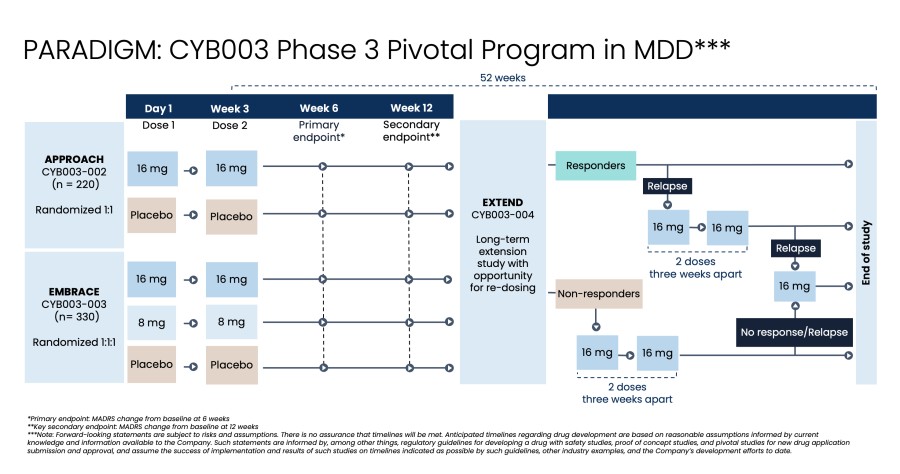
About the Phase 3 PARADIGM Pivotal Program
The Company’s Phase 3 program comprises three pivotal efficacy studies:
Pivotal study 1 (APPROACH):
APPROACH will enroll participants at 36 clinical sites across the U.S. and Europe.
Pivotal study 2 (EMBRACE):
EMBRACE is expected to enroll at 48 clinical sites, with minimal site overlap with the APPROACH study.
Pivotal study 3 (EXTEND):
Across all three studies, raters will be remote, independent, and blinded with no information on the dose received or the participant’s dosing experience. Effects during the dosing session will be firewalled to ensure that the study team stays blinded.
“Our unique Phase 3 pivotal program design has been informed by the impressive Phase 2 (four-month) data showing rapid, robust improvements in symptoms of depression with a single dose of CYB003, and durable effects four months after two doses with a 75% remission rate in the 16mg dose group. For our pivotal program, we have preserved the two-dose regimen used in our Phase 2 study, given the strong durability results seen to date,” said Amir Inamdar, Chief Medical Officer of Cybin. “The need for improved treatments for mental health disorders has never been greater. We believe that our Phase 3 program can build on the positive results demonstrated in Phase 2 to-date and could potentially lead to the approval and commercialization of a novel treatment modality whose effects are consistent and durable for patients with MDD.”
Positive Phase 2 Four-Month Efficacy Data for CYB003 in MDD
Safety and tolerability:
Upcoming Clinical Milestones and Future Studies Across Cybin’s Pipeline:
CYB003 - Deuterated Psilocin Program3
CYB004 – Deuterated DMT Program3
Second Quarter Fiscal Year 2025 Financial Information
About Cybin
Cybin is a late-stage breakthrough neuropsychiatry company committed to revolutionizing mental healthcare by developing new and innovative next-generation treatment options to address the large unmet need for people who suffer from mental health conditions.
With industry leading proof-of-concept data, Cybin is working to change the mental health treatment landscape through the introduction of intermittent treatments that provide long lasting results. The Company is currently developing CYB003, a proprietary deuterated psilocin program, in Phase 3 development for the adjunctive treatment of MDD and CYB004, a proprietary deuterated DMT program in a Phase 2 study for generalized anxiety disorder. The Company also has a research pipeline of investigational, 5-HT-receptor focused compounds.
Founded in 2019, Cybin is operational in Canada, the United States, the United Kingdom, the Netherlands and Ireland. For company updates and to learn more about Cybin, visit www.cybin.com or follow the team on X, LinkedIn, YouTube and Instagram.
Notes:
Cautionary Notes and Forward-Looking Statements
Certain statements in this news release relating to the Company are forward-looking statements and are prospective in nature. Forward-looking statements are not based on historical facts, but rather on current expectations and projections about future events and are therefore subject to risks and uncertainties which could cause actual results to differ materially from the future results expressed or implied by the forward-looking statements. These statements generally can be identified by the use of forward-looking words such as “may”, “should”, “could”, “intend”, “estimate”, “plan”, “anticipate”, “expect”, “believe” or “continue”, or the negative thereof or similar variations. Forward-looking statements in this news release include statements regarding the Company’s plan to enroll participants in its Phase 3 trials of CYB003; release topline results from the APPROACH study in 2026; initiate the EMBRACE study in the first half of calendar 2025; initiate the EXTEND study 12 weeks following commencement of the APPROACH and EMBRACE studies, respectively; release of 12-month efficacy data from Phase 2 CYB003 study in Q4 2024; release of Phase 2 topline data for CYB004 in the first quarter of calendar 2025; enroll 48 clinical sites for the EMBRACE study; the exercise in full of warrants issued pursuant to certain of the Company’s offerings; and the Company’s proprietary drug discovery platforms, innovative drug delivery systems, novel formulation approaches and treatment regimens for mental health disorders.
These forward-looking statements are based on reasonable assumptions and estimates of management of the Company at the time such statements were made. Actual future results may differ materially as forward-looking statements involve known and unknown risks, uncertainties, and other factors which may cause the actual results, performance, or achievements of the Company to materially differ from any future results, performance, or achievements expressed or implied by such forward-looking statements. Such factors, among other things, include: implications of the spread of a pandemic on the Company's operations; fluctuations in general macroeconomic conditions; fluctuations in securities markets; expectations regarding the size of the psychedelics market; the ability of the Company to successfully achieve its business objectives; plans for growth; political, social and environmental uncertainties; employee relations; the presence of laws and regulations that may impose restrictions in the markets where the Company operates; and the risk factors set out in each of the Company's management's discussion and analysis for the three and six month periods ended September 30, 2024 and the Company’s annual information form for the year ended March 31, 2024, which are available under the Company's profile on www.sedarplus.ca and with the U.S. Securities and Exchange Commission on EDGAR at www.sec.gov. Although the forward-looking statements contained in this news release are based upon what management of the Company believes, or believed at the time, to be reasonable assumptions, the Company cannot assure shareholders that actual results will be consistent with such forward-looking statements, as there may be other factors that cause results not to be as anticipated, estimated or intended. Readers should not place undue reliance on the forward-looking statements and information contained in this news release. The Company assumes no obligation to update the forward-looking statements of beliefs, opinions, projections, or other factors, should they change, except as required by law.
Cybin makes no medical, treatment or health benefit claims about Cybin’s proposed products. The U.S. Food and Drug Administration, Health Canada or other similar regulatory authorities have not evaluated claims regarding psilocin, psychedelic tryptamine, tryptamine derivatives or other psychedelic compounds. The efficacy of such products has not been confirmed by approved research. There is no assurance that the use of psilocin, psychedelic tryptamine, tryptamine derivatives or other psychedelic compounds can diagnose, treat, cure or prevent any disease or condition. Rigorous scientific research and clinical trials are needed. If Cybin cannot obtain the approvals or research necessary to commercialize its business, it may have a material adverse effect on Cybin’s performance and operations.
Neither the Cboe Canada nor the NYSE American LLC stock exchange have approved or disapproved the contents of this news release and are not responsible for the adequacy and accuracy of the contents herein.
| Last Trade: | US$9.44 |
| Daily Change: | -0.04 -0.42 |
| Daily Volume: | 16,278 |
| Market Cap: | US$188.610M |
December 10, 2024 November 18, 2024 November 14, 2024 October 24, 2024 September 19, 2024 | |

Cue Biopharma is developing the first-ever class of therapeutics for the treatment of cancer that mimic the natural signals, or “Cues”, of the immune system. This novel class of injectable biologics selectively engages and modulates tumor-specific T cells directly within the patient’s body to transform...
CLICK TO LEARN MORE
Recursion Pharmaceuticals is a clinical stage TechBio company leading the space by decoding biology to industrialize drug discovery. Enabling its mission is the Recursion OS, a platform built across diverse technologies that continuously expands one of the world’s largest....
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB