RESEARCH TRIANGLE PARK, N.C., Jan. 23, 2023 (GLOBE NEWSWIRE) -- Asensus Surgical, Inc. (NYSE American: ASXC), a medical device company that is digitizing the interface between the surgeon and the patient to pioneer a new era of Performance-Guided Surgery™, today announced receipt of CE Mark for an expansion of machine vision capabilities on the previously cleared Intelligent Surgical Unit™ (ISU™). With this CE Marking, the expanded ISU capabilities are now commercially available across all of the Company’s key geographies, including the European Union, Japan, and the U.S. In addition, this approval included a review of the Senhance® Surgical System platform, making Senhance one of the first robotic surgical systems to be approved through the new, more rigorous EU Medical Device Regulation, or MDR, process.
“We are thrilled to be able to offer these ground-breaking ISU capabilities to surgeons in the EU. Surgeon feedback from the expanded feature set across the U.S. and Japan has been tremendous, and we look forward to partnering with new and existing customers to help bring advanced real-time intraoperative digital tools into operating rooms throughout Europe,” said Anthony Fernando, Asensus Surgical President and CEO. “This is a significant milestone for the Company as our filing included a review of the Senhance Surgical System, both software and hardware, under the new, stricter EU MDR process. The fact that Senhance was one of the first robotic platforms to be cleared through the new process, in just under one year, is a testament to both the quality of our team and our technology.”
The Senhance Surgical System is powered by the Intelligent Surgical Unit, enabling real-time surgical image analytics and machine vision-driven control of the camera.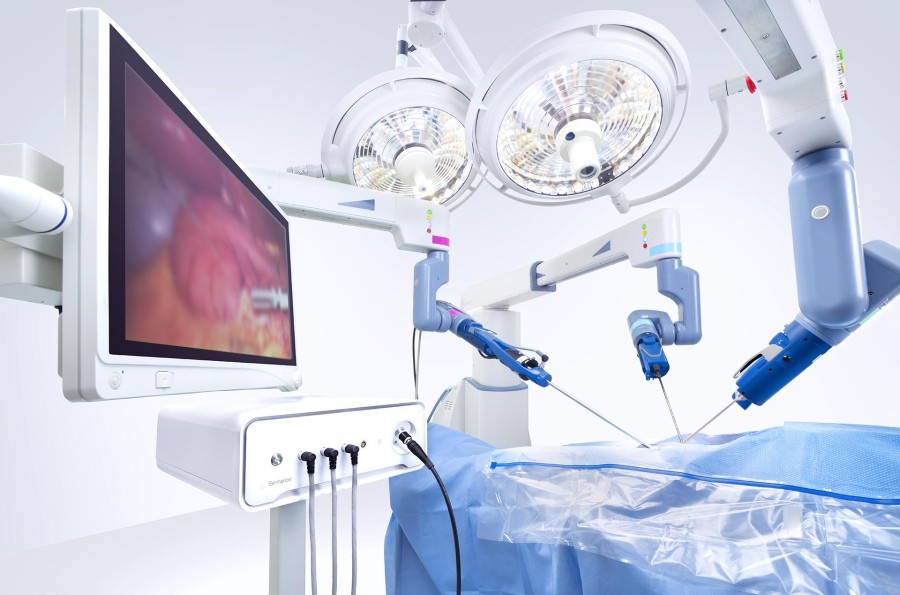
The newest ISU features introduce more advanced features including: 3D measurement, digital tagging, image enhancement, and enhanced intraoperative camera control based on real-time data.
“I have used the first generation of the ISU with the Senhance Surgical System which seamlessly supports human-machine interaction and endoscopic camera control. This second generation of ISU features will bring surgeon support to a new level,” said Prof. (Saitama Med. Univ.) Dr. med. Dietmar Stephan of St. Marien-krankenhaus Siegen in Germany. The real-time measurement tools have broad applicability in surgery, and digital tagging will support communication between console and tableside indicating critical structures, e.g. clipping points or staple lines. With these new ISU features, I expect that the Senhance Surgical System will be a technology leader in the field of augmented intelligence in surgical robotics."
The approval of these expanded augmented intelligence features demonstrates the Company's commitment to delivering on the promise of Performance-Guided Surgery. The Company believes these additional features will provide meaningful support for surgeons across a range of specialties and procedures. Additionally, the foundational elements of these features will enable the Company to continue introducing novel augmented intelligence features in the future by leveraging the vast capabilities and potential of the ISU.
About Asensus Surgical, Inc.
Asensus Surgical, Inc. is digitizing the interface between the surgeon and patient to pioneer a new era of Performance-Guided Surgery by unlocking clinical intelligence for surgeons to enable consistently superior outcomes and a new standard of surgery. This builds upon the foundation of Digital Laparoscopy with the Senhance Surgical System powered by the Intelligent Surgical Unit (ISU) to increase surgeon control and reduce surgical variability. With the addition of machine vision, augmented intelligence, and deep learning capabilities throughout the surgical experience, we intend to holistically address the current clinical, cognitive and economic shortcomings that drive surgical outcomes and value-based healthcare. Learn more about Performance-Guided Surgery and Digital Laparoscopy with the Senhance Surgical System here: www.senhance.com. Now available for sale in the US, EU, Japan, Russia, and select other countries. For a complete list of indications for use, visit: www.senhance.com/indications. For more information, visit www.asensus.com.
Follow Asensus
Email Alerts: https://ir.asensus.com/email-alerts
LinkedIn: https://www.linkedin.com/company/asensus-surgical-inc
Twitter: https://twitter.com/AsensusSurgical
YouTube: https://www.youtube.com/c/transenterix
Vimeo: https://vimeo.com/asxc
TikTok: https://www.tiktok.com/@asensus_surgical
Forward-Looking Statements
This press release includes statements relating to the Senhance Surgical System and the receipt of CE Mark for an expansion of machine vision capabilities on the ISU. These statements and other statements regarding our future plans and goals constitute "forward looking statements'' within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, and are intended to qualify for the safe harbor from liability established by the Private Securities Litigation Reform Act of 1995. Such statements are subject to risks and uncertainties that are often difficult to predict, are beyond our control and which may cause results to differ materially from expectations and include whether we will be able to partner with new and existing customers to help bring advanced real-time intraoperative digital tools into operating rooms throughout Europe; whether the second generation of ISU features will bring surgeon support to a new level; whether digital tagging will support communication between console and tableside indicating critical structures; whether with the new ISU features, the Senhance Surgical System will be a technology leader in the field of augmented intelligence in surgical robotics; whether the additional features of the ISU will provide meaningful support for surgeons across a range of specialties and procedures and whether the foundational elements of these features will enable Asensus to continue introducing novel augmented intelligence features in the future by leveraging the vast capabilities and potential of the ISU. For a discussion of the risks and uncertainties associated with the Company’s business, please review our filings with the Securities and Exchange Commission (SEC), including our Annual Report on Form 10-K for the year ended December 31, 2021, filed with the SEC on February 28, 2022 and our other filings we make with the SEC. You are cautioned not to place undue reliance on these forward looking statements, which are based on our expectations as of the date of this press release and speak only as of the origination date of this press release. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise.
INVESTOR CONTACT:
Mark Klausner or Mike Vallie, 443-213-0499
This email address is being protected from spambots. You need JavaScript enabled to view it.
MEDIA CONTACT:
Isabella Rodriguez, 708-833-1572
CG Life
This email address is being protected from spambots. You need JavaScript enabled to view it.

| Last Trade: | US$0.35 |
| Daily Volume: | 0 |
| Market Cap: | US$94.920M |
August 13, 2024 July 24, 2024 July 23, 2024 July 16, 2024 | |

Terns Pharmaceuticals is a clinical-stage biopharmaceutical company developing a portfolio of small-molecule product candidates to address serious diseases, including oncology and obesity. Terns’ pipeline contains three clinical stage development programs including GLP-1 receptor...
CLICK TO LEARN MORE
Compass Therapeutics is a clinical-stage, oncology-focused biopharmaceutical company developing proprietary antibody-based therapeutics to treat multiple human diseases. The company's scientific focus is on the relationship between angiogenesis, the immune system, and tumor growth...
CLICK TO LEARN MOREEnd of content
No more pages to load
COPYRIGHT ©2023 HEALTH STOCKS HUB